Copy link
Anesthesia Considerations for Burn Surgery
Last updated: 12/21/2023
Key Points
- The anesthetic management of burn patients poses several challenges, including potentially difficult airway, fluid and transfusion management, and temperature regulation.
- Altered pharmacokinetics and pharmacodynamics affect commonly used anesthetics, analgesics, and neuromuscular blocking agents (NMBA).
- Burn patients are more resistant to nondepolarizing NMBAs, often requiring higher and more frequent dosing.
- Succinylcholine is contraindicated up to one year after major burns due to the risk of life-threatening hyperkalemia.
Introduction
- Burns are second only to motor vehicle accidents as the leading source of accidental death.
- Three risk factors predictive of increased mortality from burns include age greater than 60 years, more than 40% total body surface area (TBSA) burns, and inhalation injury.
- Children, due to an increased body surface area to body mass ratio, and the elderly, due to thinner skin, are both at greater risk for major burn injuries.
- Data exist linking improved outcomes from major burns with early referral to a burn center, especially when 2nd or 3rd-degree burns involve more than 20% TBSA.
- See the OA summary on Burn Injuries: Initial Evaluation and Management for the classification of burns, pathophysiological changes, and the initial management of burn injuries. Link This summary will focus on the anesthetic management of burn surgery.
Anesthetic Management
- Early excision of necrotic tissue with temporary or permanent coverage of the open areas decreases the chance of wound colonization and systemic sepsis and has become the standard of care.1
- Burn patients are frequently brought to the operating room (OR) for excision of necrotic tissue, debridement, and skin grafting.
Preoperative Evaluation
- In the preoperative period, special attention must be paid to hemodynamic stability, potential for respiratory compromise and/or inhalational injury.
In addition to assessing the standard components of the preoperative history and physical examination, the preoperative evaluation should additionally focus on details, including the following:1,2- Airway patency
- Adequacy of resuscitation
- Mechanism and extent of the burn injury
- Elapsed time from the injury
- Magnitude of the surgical plan
- Age of the patient
- Associated injuries
- Hematologic issues
- Presence of infection
- Coexisting diseases and presence of organ dysfunction
- Vascular access
- Mental status and pain control
Intraoperative Management
Monitoring
- Staples may be needed to affix electrocardiogram electrodes to the chest or extremities.
- Alternative sites for pulse oximetry include the ear, nose, or tongue.
- An arterial line should be considered if it is not possible to use the noninvasive blood pressure cuff on the extremities or if there is extensive blood loss.
- Accurate temperature monitoring is essential (esophageal, nasal, bladder).
- Neuromuscular blockade monitoring is especially important due to altered dose requirements (see below).
- Intra-arterial waveform analysis can be helpful in providing information about pulse-pressure variation, stroke volume, and cardiac output that can guide resuscitative efforts.1
- Transesophageal echocardiography may also be considered for further evaluation of left ventricular end-diastolic volume for resuscitative purposes, though no studies exist regarding sufficient endpoints with this monitoring method. However, there may still be utility for its use to assess for cardiac-related issues contributing to persistent hemodynamic compromise.1,3
Vascular Access
- An edematous patient can pose technical challenges for peripheral vascular access. Ultrasound guidance should be considered.
- Central venous access can carry additional risks, given the increased risk for bloodstream infections. In addition to the internal jugular and subclavian veins, the femoral veins are commonly used.1
Induction
- Induction of general anesthesia is often accomplished with ketamine or etomidate during the acute resuscitative phase in order to preserve hemodynamic stability.
- Persistently high levels of catecholamines in patients with major burns result in desensitization and downregulation of beta-adrenoreceptors. As a result, direct myocardial depressant effects of ketamine can manifest.1
Airway Management
- Airway assessment should include any pre-existing airway abnormalities, presenting airway injury, and signs of glottic obstruction.2
- Burns to the neck or face can lead to difficulty with mask ventilation either due to serous draining from the burn site or friability of the skin itself. Patients may also present with dressings over their face and head, making noninvasive ventilation more difficult.
- Scarred tissue can result in contractures, leading to limited mouth opening and neck extension, which leads to decreased visualization by direct laryngoscopy. A video laryngoscope should be readily available.
- A smaller diameter endotracheal tube (ETT) should be used if there are concerns for inhalational injury or airway edema.
- Securing the ETT with tape will likely not be feasible either due to serous fluid at the healing burn site or due to lubricants applied to the face. Thus, ETT cloth ties or a specialized ETT tie harness should be used.
- If there is concern for a difficult airway, advanced airway techniques should be considered, including awake fiberoptic intubation, or preoperative tracheostomy can be performed.2
Maintenance
- The choice of volatile anesthetic does not appear to influence the outcome in burned patients.
- Burn patients will require larger doses of neuromuscular blockade due to resistance to nondepolarizing NMBAs.1,2
Ventilatory Management
- Findings from the Acute Respiratory Distress Network trial have become the standard of care for burn patients with acute lung injury (using tidal volumes less than 6 mL/kg and plateau pressures less than 30 cm H2O.)1
- Higher respiratory rates are often needed because of hypermetabolic states and increased CO2 production.
Fluid Resuscitation
- Delayed or inadequate fluid replacement leads to hypovolemia, tissue hypoperfusion, shock, and multiorgan failure.
- Resuscitation endpoints include:3
- Urine output ≥ 0.5ml/kg/hr (adults), ≥ 1mL/kg/hr (pediatric)
- Normotensive
- Central venous pressure 3-8mmHg
- Fractional extraction of sodium <1%
- BUN/Cr ratio ≥ 20
- Echo with normal stroke volume and ejection fraction
- Base deficit <5
- Initial phase: Isotonic crystalloids should be used for the initial resuscitation and then transitioned to colloids. This was classically done after 24 hours, but the trend now is to incorporate colloids earlier.2,3
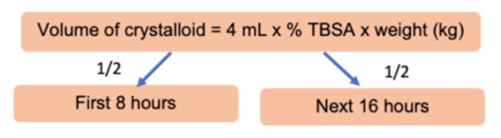
Parkland formula
-
- The use of vasoconstrictors should be minimized during the perioperative period due to concerns for inducing ischemia at the burn graft site.
- When considering the volume of fluids needed, several factors should be considered, including the magnitude of burn excisions, volume of blood loss, burn depth, specific hemostatic techniques used, and surgical administration of tumescent fluid.
- Adverse effects of large-volume crystalloid resuscitation include exacerbation of generalized edema, pleural and pericardial effusions, intestinal ileus, abdominal or limb compartment syndrome, acute respiratory distress syndrome, etc.
- After the initial phase, much effort is made to restrict fluids and administer diuretics to hasten the reduction of resultant edema. The use of colloids can help limit the amount of fluid needed to maintain preload.
Transfusion Management
- A restrictive transfusion strategy is recommended in hemodynamically stable patients without massive blood loss. A threshold of hemoglobin of less than 7-8 is often used for transfusion.4
- Blood component therapy should be reserved for patients with a demonstrated physiologic need, but anticipation of continued blood loss may indicate earlier transfusion.
- It is estimated that 2.6% of an adult patient’s total blood volume is lost (or 3.4% in children) for each 1% of burn wound excised or autograft harvested.1
- Techniques to minimize transfusion include the application of topical thrombin, the use of tourniquets, topical or subcutaneous injection of vasoconstrictors, and the use of staged procedures.
Temperature Management
- Hypothermia increases the risk of coagulopathy, infection (sepsis), and mortality.1
- Accelerated evaporative, convective, and radiative heat loss occurs through the injured skin. This is exacerbated by prolonged exposure of a large surface area to ambient temperature in the OR.
- Loss of heat is more pronounced in children with their higher surface area-to-volume ratio.
- Therapies to maintain normothermia include convective warming devices, warmed intravenous fluids, ambient room temperature ≥ 25°C, and minimizing exposed body surface area.
Pain Control
- Opioids are the mainstay of therapy in burn patients. Acute tolerance may develop after only 2 weeks, which is also complicated by opioid-induced hyperalgesia.1
- Patient-controlled analgesia has been shown to be a safe and effective method of opioid delivery.
- Multimodal pain strategies incorporating acetaminophen, ketamine, dexmedetomidine, clonidine, and methadone are useful.
- Opioid and sedative infusions should be continued intraoperatively. Intraoperative analgesia can be achieved by increasing the infusions or using additional opioids.5
- Regional anesthesia:
- Because of the heightened levels of pain at the donor site, compared to the recipient site, regional anesthesia can be a useful adjunct.
- Techniques of regional anesthesia include tumescent local anesthesia injected into the donor site, truncal blocks, peripheral nerve blocks, and neuraxial anesthesia.1,5
- Truncal blocks include transversus abdominal plane and paravertebral blocks.
- Peripheral nerve blocks may include the lateral femoral cutaneous nerve block. This purely sensory block is often performed because the lateral thigh is frequently chosen for split-thickness skin grafts. A fascia iliaca block may be considered if analgesia is needed for the anterior and medial thigh for skin harvesting.
- Spinal and epidural anesthesia may also be considered. There have been no reports of increased rates of epidural abscess in burn patients with epidural catheters in place. However, any regional anesthesia technique must consider burn involvement at the given site.1
- Posttraumatic stress disorder has been reported to occur in up to 30% of patients with severe burn injury, often developing in the setting of inadequate treatment of anxiety and pain. Nonpharmacologic therapies, including cognitive behavioral therapy, hypnosis, and virtual reality have been used successfully in the treatment of not only the pain associated with burns but also, its long-term psychological effects.5
Nutrition
- The hypermetabolic response following burn injury is more severe and sustained than any other form of trauma.1
- Fasting times and holding tube feeds should be limited.
- Continuing enteral feeding during surgery beyond the pylorus has been successful, provided the airway is secured via a cuffed ETT or tracheostomy.
Postoperative Mechanical Ventilation
- More often used for patients with inhalation burn injury, especially with developing ARDS. Also, postoperative ventilation is continued if there is an increased risk of ongoing bleeding or frequent returns to the OR, risk for graft disruption due to movement (i.e., delicate sheet grafting to face and neck).
Pharmacologic Considerations
- Plasma protein loss through injured skin and further dilution of plasma proteins by resuscitation fluids decrease the albumin concentration.
- There is an increase in the volume of distribution of most commonly used medications (propofol, fentanyl, muscle relaxants).
- During the acute injury phase, decreased cardiac output and resulting decreased renal and hepatic blood flow reduces the elimination of some drugs by the kidney and liver.
- In the hyperdynamic phase, there is increased cardiac output and blood flow to the kidneys and liver. Therefore, there is increased clearance of some drugs, requiring higher doses.
- Hepatic enzyme activity is often altered, resulting in impaired phase 1 reactions.
Neuromuscular Blocking Agents
- Burn patients exhibit resistance to all nondepolarizing NMBA due to upregulation of nicotinic acetylcholine receptor subunits, increased binding to alpha-1-acid glycoproteins, and enhanced renal and hepatic elimination of NMBAs.1,3
- The response is proportional to burn size, and the peak resistance occurs 5-6 weeks after the injury.
- It is often necessary to increase the dose and frequency of administration of nondepolarizing NMBAs.
- The dosing and timing of administration of reversal agents (anticholinesterase agents or sugammadex) should not be altered.
- Succinylcholine should be avoided after the first 48 hours, and for at least 1 year after the burn injury, due to the risk of acute severe hyperkalemia and life-threatening arrhythmias. The hyperkalemia results from a significant increase in the number of extra junctional acetylcholine receptors.1
Opioids
- There is an increased opioid requirement in burn-injury patients.5
- Doses are often reduced during the acute resuscitative phase as metabolic clearance may be impaired by liver dysfunction.
- In the subsequent recovery phase, opioid requirements often increase due to their substantially expanded volumes of distribution, as well as significant patient tolerance due to prolonged opioid therapy.1
Propofol
- Patients may require larger bolus doses and/or increased infusion rates of propofol to attain or maintain therapeutic plasma drug concentrations.1
Ketamine
- Ketamine should be considered in the approach to multimodal analgesia in the burn patient who has likely developed tolerance to opioids.6
- This can be especially useful for brief procedures, including dressing or line changes, or if one prefers to maintain spontaneous ventilation in the burn patient as opposed to manipulating the airway. Due to increased secretion production associated with ketamine, glycopyrrolate coadministration should be considered.1,6
- There may also be potential anti-inflammatory impact in burn patients.1
- Due to an upregulation of N-methyl-D-aspartate (NMDA) receptors in the spinal cord of burn patients, there is a resultant increase in requirement.1
Methadone
- Through its antagonism of the NMDA receptor, methadone can be considered in patients with opioid-induced hyperalgesia or as an adjunct to limit opioid administration in patients with difficult-to-control pain.5
Dexmedetomidine
- Dexmedetomidine infusions can be used to provide sedation for minor procedures without producing the side effects seen with opioids, including itching and respiratory depression.6
- It is also useful as an adjunct for procedures in the OR as an opioid-sparing strategy.
- As hypotension can be seen with infusions, dexmedetomidine should not be used in hemodynamically unstable patients.
References
- Bittner EA, Shank E, Woodson L, Martyn JA. Acute and perioperative care of the burn-injured patient. Anesthesiology. 2015;122(2):448-64. PubMed
- Anderson TA, Fuzaylov G. Perioperative anesthesia management of the burn patient. Surg Clin North Am. 2014;94(4):851-61. PubMed
- Pham TN, Cancio LC, Gibran NS. American Burn Association. American Burn Association Practice Guidelines Burn Shock Resuscitation. J Burn Care Res. 2008;29(1):257-66. PubMed
- Palmieri TL, Holmes JH, Arnoldo B, et al. Transfusion requirement in burn evaluation (TRIBE): A multicenter randomized prospective trial of blood transfusion in major burn injury. Ann Surg. 2017;266(4):595-602. PubMed
- Romanowski KS, Carson J, Pape K, et al. American Burn Association guidelines on the management of acute pain in the adult burn patient: A review of the literature, a compilation of expert opinion, and next steps. J Burn Care Res. 2020:41(6):1129-51. PubMed
- Griggs C, Goverman J, Bittner EA, et al. Sedation and pain management in burn patients. Clin Plast Surg. 2017;44(2):535-40. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.