Copy link
Acute Ischemic Stroke: Acute Evaluation and Treatment, Hemodynamic Goals, and Intraoperative Management
Last updated: 04/20/2023
Key Points
- For a patient with suspected acute ischemic stroke (AIS), immediate assessment in a specialized stroke center capable of delivering time-sensitive reperfusion therapies offers the best opportunity to prevent death and disability.
- A multidisciplinary approach allows emergent treatment with intravenous thrombolysis (IVT) and/or endovascular thrombectomy (EVT) in eligible patients in an effective manner.
- Optimal hemodynamic support during anesthetic management improves outcomes.
- The Society for Neuroscience in Anesthesiology and Critical Care (SNACC) has developed guidelines for perioperative, intraoperative, and postoperative management.
Acute Evaluation
- Time-sensitive reperfusion interventions including IVT and/or EVT in selected AIS patient populations improve outcomes.1
- Initial evaluation involves hemodynamic and respiratory stabilization, evaluation of blood glucose level (BGL), neurological examination, and exclusion of intracerebral hemorrhage (ICH) with noncontrast computed tomography (NCCT).1
- The use of a stroke severity rating scale, preferably the National Institutes of Health Stroke Scale (NIHSS), is recommended.1
- The NIHSS is a validated scoring system to identify and grade neurological deficits in the acute setting.
- The NIHSS is most sensitive in the detection of dominant hemispheric middle cerebral artery (MCA) syndromes.2
- Clinical features of posterior circulation stroke are not extensively evaluated, leading to lower NIHSS scores in this patient population.3
- All patients with suspected AIS should receive emergent brain imaging evaluation before initiating any specific therapy to treat AIS.
- NCCT is effective to exclude ICH before IVT administration; IVT should be initiated without delay of additional neuroimaging.
- For patients who otherwise meet criteria for EVT, vessel imaging of the intracranial and extracranial arteries is recommended.
- When selecting patients with AIS within 6 to 24 hours of last known normal (LKN) who have large vessel occlusion (LVO) in the anterior circulation, obtaining computed tomography perfusion (CTP) or magnetic resonance imaging (MRI) is recommended to aid in patient selection for EVT.
- This is applicable only when patients meet other eligibility criteria from randomized controlled trials that showed benefit from EVT in the extended time window.1
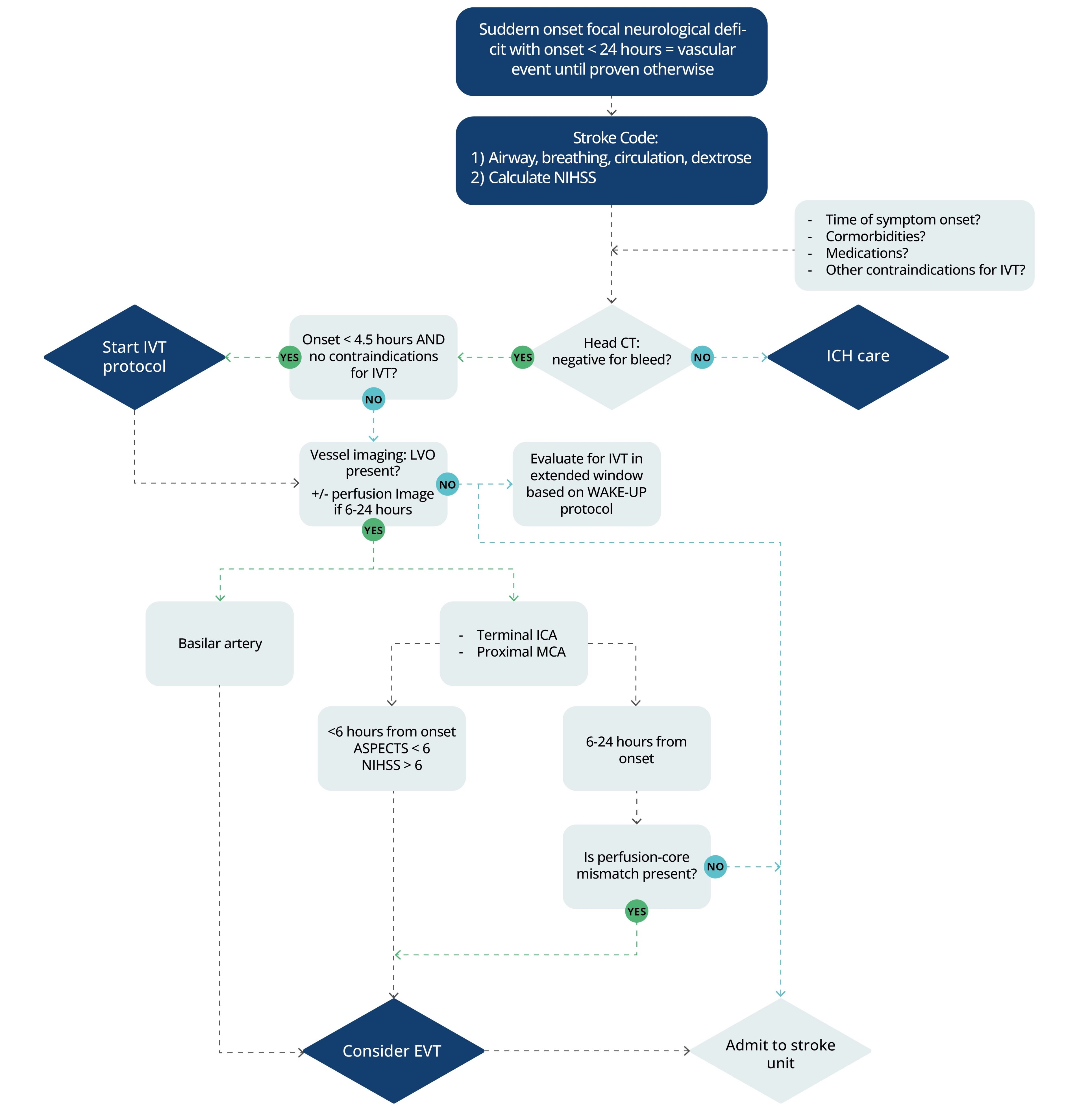
Figure 1. Acute evaluation of an acute ischemic stroke.1 Abbreviations: NIHSS, National Institutes of Health Stroke Scale; CT, computed tomography; CI, contraindications; ICH, intracerebral hemorrhage; IVT, intravenous thrombolysis; LVO, large vessel occlusion; ICA, internal carotid artery; MCA, middle cerebral artery; EVT, endovascular therapy; ASPECTS, Alberta Stroke Program Early Computed Tomography Score.
WAKE-UP protocol: For patients who awaken with unclear time of onset > 4.5 hours from last known well, may use MRI to identify diffusion-positive FLAIR-negative (MRI mismatch) lesions to select those patients who may benefit from IVT within 4.5 hours of stroke symptom recognition.
Acute Therapies
Intravenous Thrombolysis
- For AIS patients who are eligible, IVT should be administered as soon as possible (evaluated by “door-to-needle” times) even if EVT is considered.
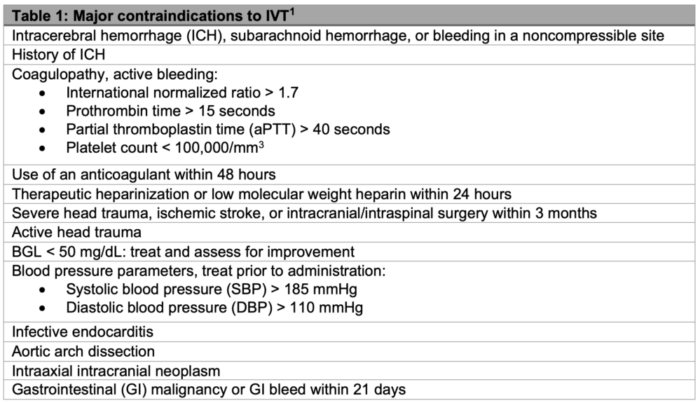
- Hypoglycemia and hyperglycemia may mimic acute stroke presentations. Therefore, blood glucose level before IVT initiation is reccomended.1
- IVT administration should not be delayed for further laboratory studies in most cases.
- Time windows for IVT:
- The Federal Drug Administration (FDA) has approved the use of IVT within 3 hours of symptom onset.
- IVT is also recommended for patients who can be treated within 3-4.5 hours of symptom onset with additional relative exclusion criterion:
- age older than 80 years;
- initial NIHSS >25; and
- history of both diabetes and prior stroke.4
- IVT can benefit AIS patients who awaken with stroke symptoms or have unclear time of onset >4.5 hours who meet certain imaging criteria.1
- Tenecteplase is emerging as an alternative to alteplase due to enhanced fibrin selectivity; however, this alternative currently lacks FDA approval.
- Alteplase dosing:
- 0.9mg/kg total dose (maximum dose of 90mg):
- 10% of total dose as bolus over one minute
- remaining 90% given over one hour via a continuous infusion1
- 0.9mg/kg total dose (maximum dose of 90mg):
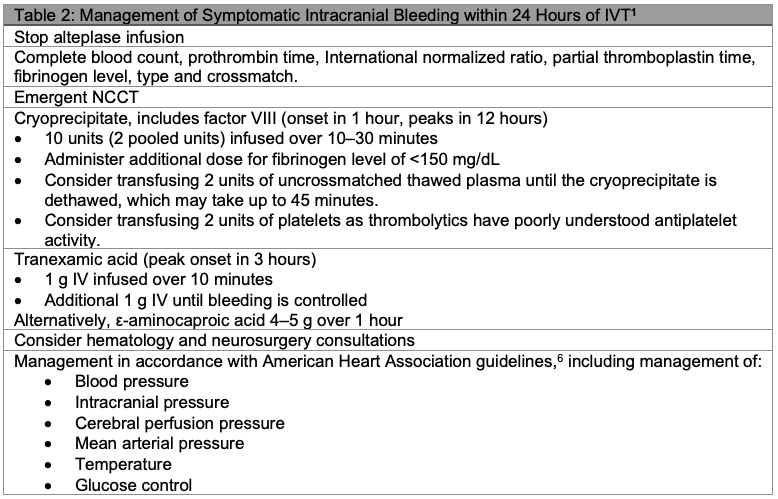
- In patients undergoing fibrinolytic therapy, physicians should be prepared to treat potential emergent adverse effects, including bleeding complications and angioedema that may cause airway obstruction.1
- Angioedema associated with IVT is treated similarly to anaphylactic reactions in respect to medical and airway management.
- Symptomatic intracranial hemorrhage (sICH) occurs in approximately 6% of cases.5
Endovascular Therapy
- For EVT, simultaneous evaluation utilizing both clinical evaluation (assessment for LVO syndrome) and advanced imaging is necessary to reduce “door-to-puncture” time.
- Current guidelines for EVT:
- Time from symptoms onset 0-6 hours:
- Benefit shown for EVT in patients with:
- Prestroke Modified Rankin Score (mRS) of 0 to 1;
- Causative occlusion of the terminal ICA and MCA M1-segment;
- Age ≥18 years;
- NIHSS score of ≥6; and
- Limited extent of early ischemic changes on NCCT.
- EVT may also be reasonable for carefully selected patients meeting the following criteria:
- Causative occlusion of additional arterial segments
- MCA M2-segment or M3-segment
- Anterior cerebral artery (ACA)
- Vertebral arteries
- Basilar artery
- Posterior cerebral artery (PCA)
- MRS >1
- Limited extent of early ischemic changes on NCCT
- NIHSS score <6
- Causative occlusion of additional arterial segments
- Time from symptoms onset 6-24 hours:
- CTP or MRI with or without perfusion is performed to aid in analysis of ischemic core and penumbra allowing for evaluation of stroke burden, tissue salvageability, and risk of hemorrhagic conversion.
- For patients with a favorable perfusion-core mismatch profile (i.e. small completed infarct and a large penumbra, or tissue at risk) and causative LVO, EVT is recommended.
- Prestroke mRS is utilized in patient selection.1
- Benefit shown for EVT in patients with:
- Time from symptoms onset 0-6 hours:
Hemodynamic Management
- Blood pressure goals are dependent upon treatment received:
- Ineligible for IVT or EVT: permissive SBP up to 220 mmHg
- IVT administered but ineligible for EVT: SBP< 180 mmHg and DPB <105 mmHg
- Following EVT with or without IVT: individual management is advised with SBP between 100-140 mmHg usually recommended dependent on degree of reperfusion.
- Preferred options to treat arterial hypertension: labetalol, nicardipine, clevidipine1
SNACC Guidelines for Anesthetic Management in EVT for AIS
- Based on limited randomized controlled trial data, there does not appear to be a difference in outcome when general anesthesia (GA) is used compared with local anesthesia with sedation.
- The need for emergent decision-making by the anesthesiologist precludes extensive preoperative evaluations and anesthesiologists should follow American Society of Anesthesiologists standards for emergency procedures.
General Recommendations for Anesthetic Management
- GA is recommended in patients who are:
- already intubated;
- agitated or unable to cooperate; and
- suffering from posterior circulation AIS.
- Both local anesthesia with sedation and GA are options for cooperative patients who can protect their airways.
- In patients receiving local anesthesia with sedation, the anesthesiologist should be prepared to rapidly convert to GA if needed.
- Anesthetic technique and pharmacological agents should be individualized in collaboration with the neuro-interventionalist.7
Management of Oxygenation and Ventilation
- Tracheal intubation is recommended for patients with:
- decreased level of consciousness;
- signs of brainstem dysfunction with compromised protective airway reflexes;
- having active nausea/vomiting before endovascular treatment;
- hypoxia or hypercarbia; and
- risk of developing airway obstruction under sedation.
- Oxygenation/ventilation recommendations:
- Supplemental oxygen should be administered and fraction of inspired oxygen (FiO2) should be titrated to maintain oxygen saturation 92-94%.
- Ventilation should be adjusted to maintain normocapnia (partial pressure of carbon dioxide (PaCO2) 35-45 mm Hg) under GA.
- Respiratory depression-induced hypercarbia should be avoided during procedural sedation.
Periprocedural Hemodynamic Management
- Hemodynamic monitoring and management should be started as soon as AIS diagnosis has been made.
- SBP should typically be maintained between 140 -180 mm Hg (fluids and vasopressors) and diastolic blood pressure <105 mm Hg.
- Rapid lowering of blood pressure during induction or in the case of arterial hypertension should be avoided for even brief periods of time. Intraoperative hypotension is a critical parameter associated with poorer outcomes.
- There is insufficient data to recommend a specific vasopressor to support blood pressure.
- Vasopressor choice should be based on individual patient characteristics.
- Blood pressure targets may be adjusted in communication with the neuro-interventionalists and neurologists (see above).
Fluid Management
- Maintaining euvolemia is recommended during EVT.
- Glucose containing fluids should be avoided unless treating BGL less than 50 mg/dL.
Temperature Management
- Maintaining target temperature between 35 – 37°C is recommended.
Intraprocedural Monitoring
- Continuous electrocardiogram (ECG), SpO2, endotracheal tube carbon dioxide, and respiratory rate monitoring is recommended.
- Blood pressure should be monitored continuously or measured noninvasively at least once every 3 minutes.
- Continuous invasive intraarterial pressure monitoring is recommended during EVT if arterial cannulation will not delay treatment.
- If feasible, the femoral artery cannulated by the neuro-interventional team may be used for continuous arterial blood pressure monitoring.
Glycemic Management
- The anesthesiologist should obtain a serum glucose value at the beginning of the procedure if it is not already available and at least once every hour thereafter.
- Serum glucose concentration should be maintained in the range of 140 to 180 mg/dL.
- Treatment for hypoglycemia should be initiated for BGL less than 50 mg/dL.7
- Protocol driven insulin infusion should be initiated for BGL more than 180 mg/dL.1
Periprocedure Management of Anticoagulation
- Anesthesiologists should be prepared to administer heparin throughout the procedure.
Management of Complications During EVT
- In the event of ICH or iatrogenic subarachnoid hemorrhage, protamine should be administered immediately to a patient who has received heparin.
- In the event of ICH, SBP is recommended to be maintained <140 mm Hg.
- Labetalol and/or nicardipine is recommended for patients with ICH to maintain SBP goals.
- If patient received IVT, follow recommendations discussed above.
Postprocedure Care
- Patients should be admitted to an intensive care unit (ICU) specializing in neurovascular care or a stroke unit after the procedure.
- Continuous hemodynamic monitoring should be continued in the ICU or stroke unit.7
References
- Powers WJ, Rabinstein AA, Ackerson T, et al. American Heart Association Stroke Council. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49: e46–e110. PubMed
- Vitti E, Kim G, Stockbridge MD, et al. Left hemisphere bias of NIH stroke scale is most severe for middle cerebral artery strokes. Front Neurol. 2022; 13: 912782. PubMed
- Alemseged F, Rocco A, Arba F, et al. Posterior National Institutes of Health Stroke Scale improves prognostic accuracy in posterior circulation stroke. J Stroke. 2022; 53:1247-55. PubMed
- de los Ríos la Rosa , Khoury J, Kissela BM, et al. Eligibility for intravenous recombinant tissue-type plasminogen activator within a population. Stroke. 2002;43 (6),1591-95. PubMed
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995; 333:1581–7. PubMed
- Greenberg SM, Ziai WC, Cordonnier C, et al. 2022 Guideline for the management of patients with spontaneous intracerebral hemorrhage: A guideline from the American Heart Association/American Stroke Association. J Stroke. 2022; 53: e282-e361. PubMed
- Talke P, Sharma D, Heyer EJ, et al. Society for Neuroscience in Anesthesiology and Critical Care Expert Consensus Statement: Anesthetic management of endovascular treatment for acute ischemic stroke. J Neurosurg Anesthesiol. 2014;26(2):95-108. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.