Copy link
Acetaminophen
Last updated: 05/09/2023
Key Points
- Acetaminophen is available in oral, intravenous (IV), and rectal formulations. It is safe for use as a first-line analgesic and antipyretic in most patient populations.
- Though adverse effects with the use of acetaminophen are uncommon with therapeutic dosing, overdosage of acetaminophen can cause clinically significant hepatic necrosis, which may progress to fulminant hepatic failure.
- Certain populations require special consideration when administering acetaminophen, including those with conditions of induction of the cytochrome P450 system and conditions of glutathione depletion. The pediatric and elderly populations may also have alterations in metabolism that warrant dose adjustment.
Structure and Mechanism of Action
- Acetaminophen, also known as paracetamol and APAP, is a weak acid and is essentially unionized at physiologic pH. After administration, it distributes to a volume of about 50L, with negligible binding to plasma proteins and tissues.1
- Despite its many years of widespread clinical use, its mechanism of action is still under investigation. Our current understanding is that it acts through several different metabolic pathways to exert its analgesic and antipyretic actions.
- Cyclooxygenase (COX) pathway (Figure 1)
- Two cyclooxygenase isozymes (COX-1 and COX-2) catalyze the conversion of arachidonic acid to prostaglandins, thromboxanes, and prostacyclin, which are mediators of inflammation, fever, and pain.2 Acetaminophen acts as a reducing agent, which facilitates the conversion of the COX enzyme from the oxidized active form to an inactive form, thereby reducing the production of the aforementioned inflammatory mediators.
- Nonsteroidal anti-inflammatory drugs (NSAIDs) also act through the COX pathway, which accounts for similar analgesic and antipyretic effects.
- The action of acetaminophen is thought to be influenced by the peroxide tone of its environment, with COX inhibition seen more so in cells with a low concentration of hydroperoxides (i.e., endothelial cells), than in cells with a high oxidant status (i.e., platelets). This mechanism contributes to acetaminophen’s relatively weak anti-inflammatory effect, as well as its lack of clinically relevant effect on platelets or coagulation.3
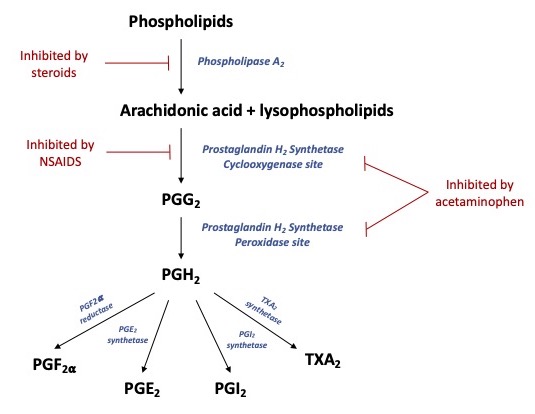
Figure 1. Schematic diagram of arachidonic acid metabolism via the COX pathway, with eventual production of prostaglandins, prostacyclin, and thromboxanes. Acetaminophen interferes with activity at both the cyclooxygenase and peroxidase sites of the prostaglandin H2 synthetase enzyme, commonly referred to as the COX enzyme. Adapted from Anderson BJ, 2008.2 Abbreviations: PGG2, prostaglandin G2; PGH2, prostaglandin H2, PGF2⍺, prostaglandin F2-alpha; PGE2, prostaglandin E2; PGI2, prostacyclin; TXA2, thromboxane A2.
- Acetaminophen is also postulated to act through interference with serotonergic descending pathways, inhibition of N-methyl-D-aspartate (NMDA)-mediated nociceptive transmission via the nitric oxide pathway, and indirect activation of cannabinoid receptors.2
- Spinal 5-hydroxytryptamine type 3 (5-HT3) receptors are thought to be involved in the antinociceptive effect of acetaminophen. Coadministration of tropisetron or granisetron (5-HT3 receptor antagonists) with acetaminophen blocked the analgesic effect in human volunteers in one study.2
- Acetaminophen may exert an inhibitory action on the synthesis of nitric oxide at the spinal level, which in turn may interfere with nociception via spinal NMDA receptor activation.2
- N-arachidonoylphenolamine (AM404) is an active metabolite of acetaminophen that has been identified in mice. AM404 appears to be an activator of one of the ligands at cannabinoid receptors, with the downstream effect of increased levels of endogenous cannabinoids.2
Metabolism
- Acetaminophen is metabolized through hepatic sulfation (~60%), glucuronidation, or oxidation, with a very small percentage undergoing direct renal elimination. The oxidation pathway occurs via the cytochrome P-450 system (CYP2E1).3
- The small proportion that undergoes cytochrome-mediated metabolism forms N-acetyl-p-benzoquinon imine (NAPQI), which is a highly reactive metabolite.3
- Detoxification of NAPQI occurs primarily via glutathione (GSH) conjugation.
- When hepatic GSH is depleted, however, NAPQI can cause direct hepatotoxicity, contributing to the toxic effects of acetaminophen overdose as described below.3
- Metabolites of acetaminophen are ultimately excreted in urine.
Dosing and Clinical Uses
- Acetaminophen is available in oral, IV, and rectal formulations.
- Oral dosing in adults is 325-650mg every 4-6h; the maximum total daily dose is 4g (2-3g/d for patients with hepatic insufficiency and/or a history of chronic alcohol use).3
- Of note, in 2011, the United States Food and Drug Administration announced a mandate to limit acetaminophen to 325 mg/tablet in combination prescription acetaminophen and opioid products. Shortly thereafter, McNeil laboratories issued packaging changes to recommend a 3g daily maximum on their Tylenol products.
- Some studies of IV and oral formulations have demonstrated opioid-sparing effects but no significant difference in opioid-related side effects or length of stay. Despite a lack of demonstration of clinically significant benefits of IV over oral administration, IV acetaminophen is a useful adjunct in the perioperative period for patients who are unable to tolerate oral administration.1
- In adults, peak plasma concentrations occur within 30-60 minutes after oral administration. Half-life is approximately 2 hours.3
- Age-related clearance changes secondary to the immaturity of metabolic pathways have implications for pediatric dosing.4
- Dosing regimens are aimed at a target steady-state concentration of 10 mg/L, which has been associated with adequate reduction of temperature and pain scores in children.
- This steady-state concentration can be achieved with oral dosing of 25-60 mg/kg/day in neonates, with lower dosing for premature neonates and higher for those at term, and with dosing up to 90 mg/kg/day. Dosing interval also varies by age (Table 1).
- Beyond 6 months of age, typical pediatric dosing is ~10-15mg/kg for a single dose; no more than 5 doses in 24 hours.3
- Oral and IV formulations are dosed equivalently. Rectal formulations are relatively less bioavailable, thus requiring higher doses of up to 30 mg/kg/day in premature neonates and up to 120 mg/kg/day in infants older than 6 months of age.4
- Some recommend the use of a loading dose before initiation of a maintenance dosing regimen to more quickly achieve therapeutic plasma concentration.4
- E.g., 30 mg/kg for the initial dose, followed by 15 mg/kg for each subsequent dose.
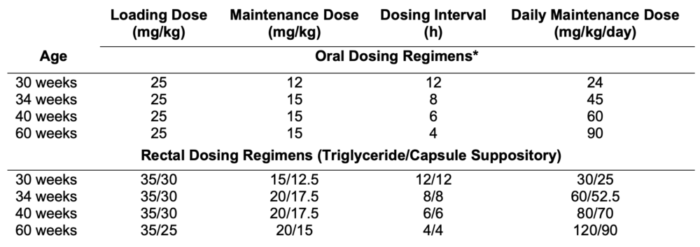
Table 1. Dosing guidelines for goal steady-state concentrations of 10mg/L. Adapted from Anderson BJ, et al., 2002.4* Oral dosing regimens can be applied to IV dosing.
Side Effects and Special Considerations
- With therapeutic dosing, acetaminophen has no clinically relevant effects on the cardiovascular and respiratory systems, platelets or coagulation, making it particularly valuable for patients with bleeding contraindications or in whom aspirin is contraindicated.3 Further, gastrointestinal adverse effects are less common than with traditional NSAIDs (Table 2).
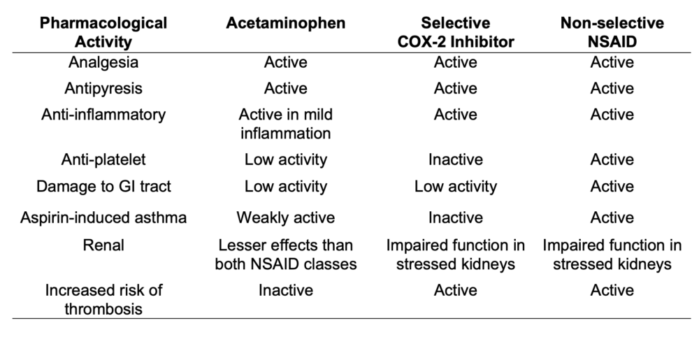
Table 2. Summary of the pharmacological and clinical activity of acetaminophen and traditional NSAIDs. Abbreviations: COX-2, cyclooxygenase-2; NSAID, non steroidal anti-inflammatory drug; GI, gastrointestinal. Adapted from Graham GG, et al., 2013.1
- Overdosage of acetaminophen, however, can cause hepatic injury, which may progress to fulminant hepatic failure necessitating liver transplantation.3
- In the setting of acetaminophen overdose, hepatic glutathione (GSH) stores become depleted, and the toxic metabolite, NAPQI, is not able to be eliminated via GSH conjugation. Instead, NAPQI is able to bind covalently to hepatocytes and cause direct cytotoxicity through a variety of mechanisms, including causing dysfunction of enzymatic systems.
- Depletion of intracellular GSH stores also renders the hepatocytes susceptible to oxidative stress and apoptosis.
- In the adult population, a single ingestion of 10-15g (150-250mg/kg) can cause hepatotoxicity, and doses of 20-25g or more are potentially fatal.
- Symptoms of acetaminophen overdose/toxicity typically begin with nausea, abdominal pain, and anorexia and within 2-4 days, progress to manifestations of hepatic injury, including tender hepatomegaly, jaundice, hypoglycemia, and aberrations in coagulation.3
- If given within 4h of ingestion, activated charcoal significantly decreases acetaminophen absorption by up to 90%, and should be administered in cases of known or suspected toxic ingestion.3
- N-acetylcysteine functions by detoxifying NAPQI and helps to replete GSH stores. Timely treatment with N-acetylcysteine can help prevent or at least limit hepatic injury.
- The risk of hepatotoxicity and the potential need for treatment with N-acetylcysteine can be predicted with the use of the Rumack-Matthew nomogram (see Other Resources below).
- Certain patients may be more susceptible to hepatic injury, including those with conditions of CYP induction (e.g., chronic alcohol consumption, concomitant use of some antiepileptic and tuberculosis medications) and conditions of GSH depletion (e.g., fasting and malnutrition). In these patients, standard daily dosing is relatively contraindicated, and more common practice is to reduce the maximum daily dose to 2-3g/day.
- Acetaminophen can be administered to patients with chronic liver disease (CLD). Though there have been literature reports of patients with CLD tolerating normal adult doses of 4g per day for several consecutive days, common practice is to use a dose reduction of 2-3g per day in this population.5
- Hesitancy to prescribe acetaminophen to patients with CLD may, in fact, lead to subtherapeutic dosing and poor efficacy and/or progression to alternative analgesics, such as opioids, which have their own well-documented side effects in this patient population.
- In advanced cirrhosis, synthesis of glutathione may be impaired, so these patients may theoretically be at higher risk of toxicity, thus supporting a lower daily dose.
- Chronic alcohol use is another relative contraindication to acetaminophen use, warranting dose adjustment.5
- Ethanol is an inducer of CYP2E1, therefore increasing metabolism through the NAPQI pathway and increasing the risk of toxicity. The risk appears to be highest in the acute withdrawal phase, as enzyme activity can take between 3-8 days to return to normal.
- Chronic alcohol use is often associated with malnourishment, which is in and of itself a risk factor for acetaminophen toxicity with regular dosing secondary to a reduction in glutathione availability.
- Acetaminophen can be administered to a patient taking warfarin; however, there is a potential for potentiation of its effects on coagulation. Laboratory evaluation of coagulation parameters should be closely monitored in any patient taking warfarin for whom acetaminophen is added or removed from their medication regimen.1
- Probenecid, commonly used in the treatment of gout, has been shown to extend the half-life of acetaminophen, and it has been suggested that the maximum daily dose of acetaminophen should be reduced to 3g in patients concurrently taking probenecid.1
References
- Graham GG, Davies MJ, Day RO, et al. The modern pharmacology of paracetamol: Therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology. 2013;21(3):201-32. PubMed
- Anderson BJ. Paracetamol (Acetaminophen): Mechanisms of action. Paediatr Anaesth. 2008;18(10): 915-21. PubMed
- Grosser T, Smyth E, FitzGerald GA. Anti-inflammatory, antipyretic, and analgesic agents; Pharmacotherapy of Gout. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman and Gilman’s: The Pharmacological Basis of Therapeutics, 12e. New York, NY: McGraw-Hill Education; 2015.
- Anderson BJ, Van Lingen RA, Hansen TG, et al. Acetaminophen developmental pharmacokinetics in premature neonates and infants: A pooled population analysis. Anesthesiology. 2002;96(6): 1336-45. PubMed
- Hayward KL, Powell EE, Irvine KM, et al. Can paracetamol (acetaminophen) be administered to patients with liver impairment? Br J Clin Pharmacol. 2016;81(2) 210-22. PubMed
Other References
- The Rumack-Matthew nomogram can be used to assess the risk of hepatotoxicity after acetaminophen ingestion/overdose: Rumack BH, Peterson RC, Koch GG, Amara IA. Acetaminophen Overdose: 662 Cases with Evaluation of Oral Acetylcysteine Treatment. Arch Intern Med. 1981;141(3):380-5. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.