Copy link
Magnetic Resonance Imaging Safety
Last updated: 02/05/2024
Key Points
- Magnetic resonance imaging (MRI) machines create powerful magnetic fields that are always on and present unique challenges and safety considerations in the delivery of anesthetic care during imaging procedures.
- Metallic objects, medical devices, and implants present safety hazards in the MRI environment. All patients and personnel must complete screening procedures prior to entering zone IV (MRI room).
- Advanced resuscitation (i.e., defibrillation) is not possible in an MRI scanner. Patients must be removed from zone IV during medical emergencies to prevent the introduction of hazardous ferromagnetic materials.
MRI Zones
The American College of Radiology defines four zones relating to personnel and care provided near MRI machines.1
- Zone I: This includes all areas that are freely accessible to the public.
- Zone II: This area is the interface between the public zone I and the strictly controlled zones III and IV. Typically, patients are greeted in zone II and receive answers to their screening questions.
- Zone III: Only screened MRI patients and personnel have access to this restrictive zone. There are potentially hazardous energies (related to the MR imaging process) present.
- Zone IV: This zone is always located within zone III and houses the MRI scanner. Patients in zone IV are under constant direct supervision of radiology personnel.
Contraindications
MRI magnetic fields are always active. There are several potential dangers to patients upon interaction with ferromagnetic foreign bodies.2
- Projectile effect
- Twisting
- Burning (heating of the object)
- Artifacts (monitoring devices)
- Device malfunction (implantable cardioverter defibrillator, programmable shunt, etc.)
- Hearing loss (high ambient noise inside machine)
Absolute Contraindications3
- Cardiac implantable electronic devices (MRI conditional devices are now available)
- Implantable neuromodulation devices
- Medication infusion pumps (insulin pumps)
- Hearing aids/cochlear implants
- Intraocular metallic foreign bodies
- Catheters with metallic components (Swan-Ganz catheter)
- Ferromagnetic vascular clips/coils
- Ferromagnetic dental implants
- Artificial limbs
- Tissue expanders (breast)
- Nonremovable piercings
- Retained foreign bodies (bullets, shrapnel, welding, etc.)
Relative Contraindications
Patients presenting with the following implants or conditions require thorough evaluation before MRI, as these items may be considered relative contraindications:
- Coronary and peripheral stents (the make and model must be reviewed)
- Programmable shunts (i.e., ventriculoperitoneal shunts must be reprogrammed after scan)
- Tracheostomy tubes containing metallic components (Bivona tracheostomy tubes)
- Wire-reinforced epidural catheter
- Vagal nerve stimulator
- Intrauterine devices
- Surgical clips/wires
- Prosthesis, joint replacements
- Medication patches (must be removed)
- Tattoos (within the last six weeks or over area of interest)
- Claustrophobia
- High body mass index (consider table limitations and bore size)
- Contraindication to gadolinium contrast agents
Compatibility: Implants/Devices
The wide variety of implants available, including newer MRI conditional implants, demands that MRI care teams seek information regarding compatibility from the device manufacturer. Equipment and implants are labeled as follows:
- MRI Safe: Safe for all strengths and fields present in any commercial MRI machine
- MRI Conditional: Manufacturer’s recommendations for appropriate use should be reviewed.
- MRI Unsafe: The device cannot be utilized within zone lV of an MRI suite.
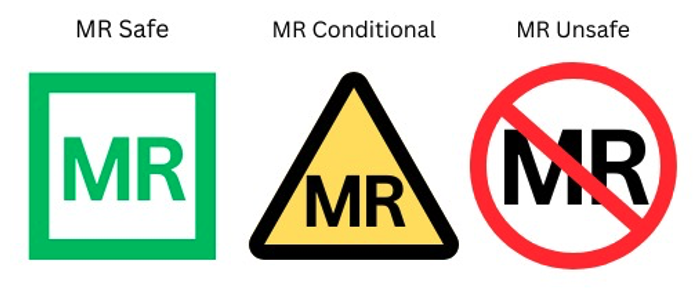
Figure 1. Labeling of portable devices in MRI environment
Physiologic Monitoring
All patients undergoing MRI should be visually monitored and able to communicate verbally with staff. Patients who are sedated, anesthetized, or unable to communicate verbally should be physiologically monitored as appropriate.4
- If a patient is given a sedative, respiratory rate and oxygenation should be monitored for apnea and desaturation.
- An anesthetized patient should be monitored using standard American Society of Anesthesiologists (ASA) monitors and more invasive methods as appropriate.5
Monitoring Safety
The limitation on ferromagnetic objects produces challenges to monitoring patients in the MRI suite:
- Electrocardiogram (ECG) limb electrodes need to be as close together as possible and in the same plane, parallel to the magnetic field, to reduce interference. All leads should be insulated from the skin, if possible, to prevent burns. Wired leads should not be used as the conductive metal can heat and burn.
- Pulse oximeters should not contain ferromagnetic substances and be placed as far as possible from the scan site.
- Capnometers are appropriate for use, noting that extended tubing may be necessary.
- Blood pressure oscillometric systems are appropriate for use. Invasive monitoring is also appropriate, noting that extended tubing may be necessary.
- Temperature probes that use ferromagnetic substances must be avoided.
Artifacts
- MRI can interfere with ECG monitoring, and conductive blood flowing through strong magnetic fields also distorts ECG signals. The most common artifact is an increase in the amplitude of the T wave. Nonspecific wave abnormalities are also possible. A baseline 12-lead ECG should be obtained before the MRI procedure if there is a concern for ST changes or arrhythmia.
- Pulse oximeters are susceptible to inference from magnetic fields and may produce inaccurate measurements or exhibit interruption in signal transmission.
- Capnometers may require lengthened tubing that can cause an elongation of the waveform upslope. The end-tidal carbon dioxide concentration may not be accurate but can be used to monitor trends and detect circuit disconnection.
- Blood pressure cuffs can disturb lightly sedated patients and interfere with MR imaging.
- Temperature probes that use thermistor or thermocouple measurements can be falsely elevated due to direct heating of the probe.
Monitoring Hazards/Complications
- Patient visibility may be limited when the patient is moved into the MRI bore. Equipment may become dislodged as the patient is moved in and out of the machine during imaging sequences.
- Communication with the patient is also challenging due to high ambient noise during the scan.
- Patient monitoring is conducted from an adjacent MRI control room using direct visualization through a window, cameras, and an audio system to communicate with the patient.
- Transient and permanent hearing loss has been reported after MRI due to high noise levels (>90dB). Headphones or earplugs should be placed on the patient and used by personnel who need to be present within the scanner.
- Temperature under anesthesia can decrease significantly, especially in young children and neonates. Heat generated by the machine can also cause hyperthermia. Longer duration of imaging has been associated with larger temperature increases in children.6
- Ferromagnetic objects can turn into projectiles upon entering MRI zone lV. If there is any uncertainty of the ferromagnetic status of an object, it should be tested with a large handheld magnet outside the scanner. When in doubt, avoiding exposure of the objects to the electromagnetic field is advisable.
- Nonferromagnetic metals can distort the images generated and heat up if in close proximity to the area to be scanned.
Radiofrequency Burns
- Inductive heating
- Changing magnetic fields present in MRI scanners can generate electrical currents.
- Heating of a resonant loop
- A coiled wire near a patient undergoing an MRI can create voltage flow and heat. For optimal safety, all loops should be removed, and padding placed between the skin and monitoring wires.
- Conductive loops can result from skin-to-skin contact (touching ankles, knees, hand-hip, or elbow-torso) and subsequently lead to burns on both surfaces.
- Antenna effect
- Elongated conductive objects such as wires (i.e., ECG leads) can heat and burn when interacting with the strong magnetic fields in the scanner.
Resuscitation
The ferromagnetic properties of the MRI scanner located in zone lV limit the equipment that can be deployed in the case of an emergency. For example, defibrillators cannot be used in zone lV. ASA guidelines published in 2015 recommend the following.5
- Immediate removal of the patient from zone lV with concurrent cardiopulmonary resuscitation to a designated area for resuscitation
- Call for help!
- The resuscitation area should be as close to zone lV as possible and have advanced resuscitation equipment such as monitors, oxygen, intubation equipment, a defibrillator, and a code cart.
Quenching
- Quenching refers to the emergency shutdown procedure of an MRI scanner. During the quench, liquid helium around the superconducting coils is rapidly expelled and dissipates the magnetic field. If not properly vented, helium gas can displace atmospheric gases in the room, presenting an asphyxiation and cold injury risk for personnel and patients in the scanner room.
- All staff and patients should be immediately evacuated from the room and oxygen masks be made available to all people in the MRI scanner if they are not able to evacuate.
- Activating the emergency shutdown of an MRI should only be done for life-threatening emergencies such as7:
- a fire in the MRI suite, to allow firefighters safe access; and
- an object pinning a person to the MRI scanner.
- Following a quench procedure, air may condense and create liquefied oxygen on surfaces. Any liquid on cold surfaces must be presumed to be liquid oxygen and treated as a potential fire hazard.
References
- Expert panel on MR safety, Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document on MR safe practices. 2013. J Magn Reson Imaging. 2013;37(3):510-30. PubMed
- Stecco A, Saponaro A, Carriero A. Patient safety issues in magnetic resonance imaging: state of the art. Radiol Med. 2007;112(4):491-508. PubMed
- Ghadimi M, Sapra A. Magnetic resonance imaging contraindications. Statpearls. Updated May 1, 2023. NCBI bookshelf. Link
- Shellock, F. Monitoring patients in the MR environment. MRIsafety.com. Accessed April 22, 2023. Link
- Practice advisory on anesthetic care for magnetic resonance imaging: an updated report by the American Society of Anesthesiologists task force on anesthetic care for magnetic resonance imaging. Anesthesiology. 2015; 122(3), 495–520. PubMed
- Madsen TW, Sørensen MK, Cromhout PF, et al. Temperature change in children undergoing magnetic resonance imaging-An observational cohort study. Paediatr Anaesth. 2022;32(7):870-9. PubMed
- Tsai LL, Grant AK, Mortele KJ, et al. A practical guide to MR imaging safety: What radiologists need to know. Radiographics. 2015;35(6):1722-37. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.