Copy link
Malignant Hyperthermia
Last updated: 06/21/2024
Key Points
- Malignant Hyperthermia (MH) is a potentially fatal syndrome of skeletal muscle hypermetabolism that occurs when a genetically susceptible patient is exposed to an anesthetic-triggering agent.
- Dantrolene is the mainstay of treatment for an MH crisis.
- Other care during a crisis is supportive. Particular concerns are hyperthermia, acidosis, hyperkalemia, and rhabdomyolysis.
Introduction
- Malignant Hyperthermia (MH) is a potentially fatal syndrome of skeletal muscle hypermetabolism that occurs when a genetically susceptible patient is exposed to an anesthetic-triggering agent.
- Incidence is difficult to assess but estimated to be 1:50,000-200,000 anesthetics with triggering agents.
- The incidence of MH is higher in the pediatric population.
Etiology
- MH susceptibility is due to a genetic variation in skeletal muscle proteins related to calcium regulation, most commonly RYR1, the ryanodine receptor.1
- Other involved genes are CACNA1S (which encodes the dihydropyridine receptor) and STAC3 (which encodes another protein involved in excitation-contraction coupling).
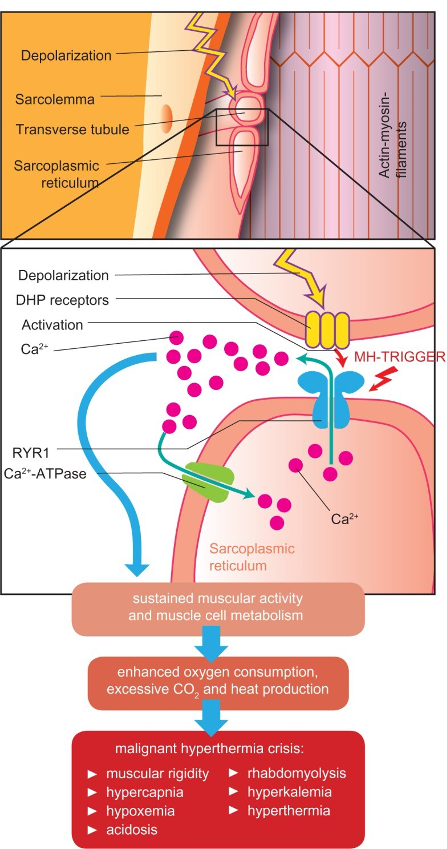
Figure 1. Pathophysiology of an MH crisis. In a normal muscle cell, depolarization travels down the t-tubule system. It activates the dihydropyridine receptor (DHP), which activates the ryanodine receptor (RYR1), a calcium channel that opens to allow calcium release from the sarcoplasmic reticulum (SR) into the cytosol. Cytosolic calcium allows muscle contraction, a process that utilizes energy and creates heat. In MH, exposure to a triggering anesthetic agent and abnormalities of the DHR or RYR1 cause a prolonged opening of RYR1 and increased intracellular calcium levels, leading to sustained muscle contraction, excess energy utilization, acidosis from anaerobic metabolism, heat production, and possibly muscle cell breakdown (rhabdomyolysis). Source: Schneiderbanger D, et al. Management of malignant hyperthermia: diagnosis and treatment. Therapeutics and Clinical Risk Management. 2014:10:355-62. CC BY NC.
- A recent study estimated the incidence of pathogenic or likely pathogenic variants of genes that confer MH susceptibility (MHS) to be greater than 1 in 800 people in the US.2
- The penetrance of MH is defined as the percentage of times an MH reaction occurs when known MHS patients are exposed to triggering agents. This is estimated to be between 5% and 40%. Many MHS patients have prior uneventful anesthetics with exposures to triggering agents.3
- Exposure to volatile agents or succinylcholine leads to an unregulated rise of myoplasmic calcium, which causes the classic symptoms of hyperthermia, hypercarbia, rigidity, and acidosis. Table 1 lists triggering agents and safe medications.
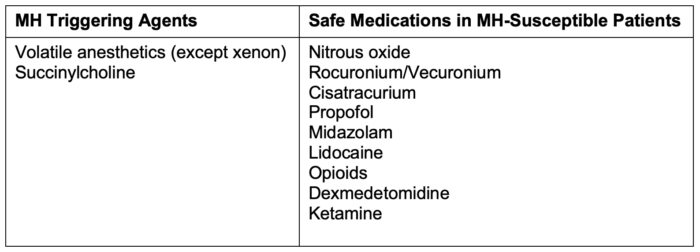
Table 1. Triggering agents and safe medications in MH susceptible patients
- There are a few congenital syndromes associated with MHS (Table 2). While Duchenne and Becker muscular dystrophy can have life-threatening hyperkalemia and rhabdomyolysis with succinylcholine and volatile agents, this is related to anesthesia-induced rhabdomyolysis (AIR), a separate process from MH.
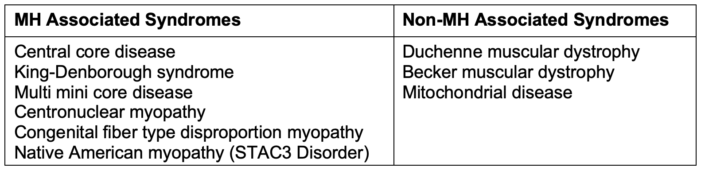
Table 2. Syndromes associated and not associated with MH
Diagnosis and Presentation of MH Crisis
- The classic presentation of MH crisis is the physiologic signs of hypermetabolism, including:
- Hypercarbia that is resistant to increases in minute ventilation. After confirming adequate ventilation, hypercarbia resistant to increases in minute ventilation should prompt a suspicion of MH.
- Sinus tachycardia
- Hypertension
- Metabolic acidosis
- Tachypnea if spontaneously breathing
- Hyperthermia can occur early or late in the course of an MH crisis. Core temperature monitoring should be initiated in all general anesthetic cases lasting longer than 30 minutes.
- Muscular signs may include masseter muscle rigidity (MMR), generalized skeletal muscle rigidity, and rhabdomyolysis (which may lead to severe hyperkalemia and arrhythmias.)
- MMR (severe and prolonged tightening of the masseter muscle that resists manual opening, so-called “jaws of steel”) can be a harbinger of an MH episode. If it occurs after administration of a triggering agent, it should prompt a search for other MH signs while stopping the triggering agents. After a severe masseter spasm, creatine kinase levels should be followed, and close observation should be considered postoperatively as rhabdomyolysis can occur.
- Other signs include possible cyanosis from increased oxygen utilization and hypotension and coagulopathy if metabolic derangements are severe.
- The differential diagnosis for MH is broad (see Table 3).
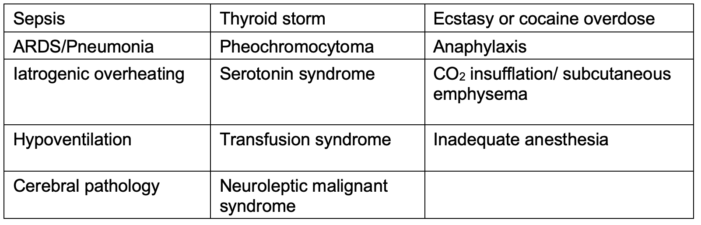
Table 3. Differential diagnosis for MH
Treatment4
- Discontinue the administration of volatile agents and succinylcholine
- Patient should be hyperventilated with 100% oxygen and intubated if not done previously.
- Call for help and the MH cart
- Call the Malignant Hyperthermia hotline (1-800-644-9737 in the United States and 001-209-417-3722 outside North America)4
- Place charcoal filters on the inspiratory and expiratory limbs of the anesthesia circuit. Increase fresh gas flows to at least 10L/min.
- Notify the surgeon to pause or complete the case as soon as possible
- Administer intravenous (IV) dantrolene rapidly through the largest bore IV available. The initial dose of dantrolene is 2.5mg/kg. Repeat as frequently as needed until there is a decrease in EtCO2 and muscle rigidity. The average effective dose is 5 mg/kg.
- If more than 10mg/kg dantrolene has been given without improvement, other diagnoses should be considered. Be aware that some formulations of dantrolene can take significant time to reconstitute an appropriate dose, while others can be reconstituted more rapidly.
- For older dantrolene preparations (Dantrolene sodium, Dantrium, Renovo), dilute each 20 mg vial with 60 mL of sterile preservative-free water; for a 70 kg patient, prepare nine 20 mg vials (Figure 2). Shake the vial to ensure an orange-colored uniform suspension.
- For Ryanodex, dilute a 250 mg vial with 5 mL of sterile preservative-free water (Figure 2)
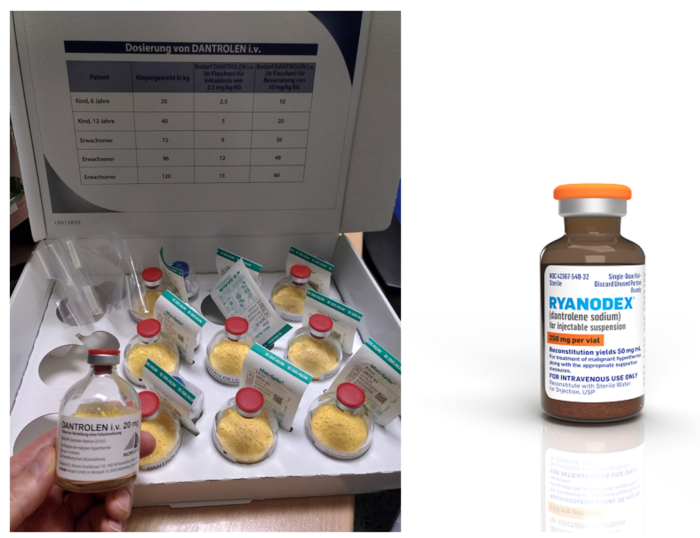
Figure 2. Dantrolene sodium formulations. The older formulation on the left (Dantrium, Revonto) contains 20mg per vial and requires 60 mL sterile water per vial to mix for a final concentration of 0.33mg/mL. The newer formulation on the right (Ryanodex) contains 250mg per vial and requires 5 mL sterile water to mix for a final concentration of 50mg/mL. Dantrolene image (left panel): Source: Pavel Dusek, Wikimedia. Link. Ryanodex image (right panel): Courtesy of Eagle Pharmaceuticals.
- Treat hyperthermia with cold IV fluids, ice packs, and cooling blankets. Discontinue when the patient’s temperature is below 38°C to avoid overcorrection hypothermia. Dantrolene will also bring down the patient’s temperature non-specifically. Therefore, a decrease in temperature alone after dantrolene administration is not diagnostic of an MH crisis.
- Obtain a blood gas to assess the degree of acidosis and hyperkalemia.
- For a base excess over –8, consider administering sodium bicarbonate 1-2 mEq/kg, with a maximum dose of 50 mEq.
- For hyperkalemia over 5.9 or less with EKG changes, consider administering calcium chloride 10-20mg/kg (max 2g initial dose) or calcium gluconate 60-100mg/kg (max 3g initial dose). Additionally, consider administering 0.1U/kg regular insulin and 0.5g/kg dextrose (10U insulin and 25g dextrose (50cc of 50% dextrose) are standard starting doses for adults).
- Can consider administering albuterol, kayexalate, or dialysis for refractory hyperkalemia.
- Treat dysrhythmias as they arise and assume hyperkalemia as the primary cause if present. Avoid calcium channel blockers with dantrolene administration.
- Insert a Foley catheter and initiate diuresis to a urine output of at least 1cc/kg/hr. Consider alkalinizing the urine with a bicarbonate infusion (1mEq/kg/hr).
- When stable, transfer the patient to the intensive care unit for at least 24 hours and continue to monitor for recurrence, rhabdomyolysis, and disseminated intravascular coagulation.
- Administer 1mg/kg dantrolene every 6 hours for 24 hours to prevent recrudescence.
- File an Adverse Metabolic Reaction to Anesthesia (AMRA) report with the North American MH Registry (https://anest.ufl.edu/namhr/).
- Refer the patient for genetic testing for MHS and make family members aware that they may be MHS. Also, referrals to a clinical geneticist and neurologist for follow-up should be considered, as MH may be the presenting sign of an underlying metabolic disease or myopathy.
Diagnosis of MH Susceptibility
- An MH crisis is a clinical diagnosis. Following a suspected MH crisis, further diagnostic tests can be pursued.
- If a patient or family member has a clinically suspicious episode of MH, diagnostic testing for MHS should be performed.
- Two current methods of diagnosis are molecular genetic testing and muscle biopsy with caffeine-halothane contracture testing (CHCT).
- Genetic testing for pathogenic variants is becoming more cost-effective and accessible. It is recommended for all patients who have had a suspicious episode of MH and their relatives. A positive finding of one of over 60 known pathogenic variants confirms MHS. A negative test does NOT preclude MH susceptibility, as all genetic variants that can confer MHS have not been elucidated.
- CHCT involves an open incision and muscle biopsy to immediately compare halothane and caffeine-induced contracture. A negative CHCT definitively rules out MH susceptibility. However, it is invasive and costly with limited availability. Currently, only three centers in the United States perform the CHCT (Wake Forest University in North Carolina, University of Minnesota, and Uniformed Services University in Maryland). As the testing must be done on freshly harvested muscle, patients must travel to the CHCT testing facility.
Prevention
- For known or suspected MHS patients, take key preventative steps.
- Place charcoal filters on the inspiratory and expiratory limbs of the anesthesia breathing circuit. When used for prevention, they last for about 12 hours.
- Consider flushing the anesthesia machine. Different manufacturers have specific recommendations for flushing their anesthesia machines. Consider changing the CO2 absorbent as well. Of note, the flush time to remove residual volatile anesthetic with charcoal filters can be as low as 90 seconds.5
- Avoid triggering agents. Removing the vaporizers and succinylcholine from the operating room is best.
- Ensure the MH kit is fully stocked and available before proceeding with the case. Please do not move the MH kit from its usual location.
References
- Pinyavat T, Girard T, and Litman RS. Malignant Hyperthermia. In Litman RS, Ambardekar A. Litman’s Basics of Pediatric Anesthesia. Third Edition. Elsevier Health Sciences; 2022: 159-166.
- Yu KD, Betts MN, Urban GM, et al. Evaluation of malignant hyperthermia features in patients with pathogenic or likely pathogenic RYR1 variants disclosed through a population genomic screening program. Anesthesiology 2024; 140 (1): 52-61. PubMed
- Ibarra Moreno CA, Hu S, Kraeva N, et al. An assessment of penetrance and clinical expression of malignant hyperthermia in individuals carrying diagnostic ryanodine receptor 1 gene mutations. Anesthesiology. 2019;131(5):983-91. PubMed
- Malignant Hyperthermia Association of the United States. Accessed April 5th, 2024. Link
- Kim TW, Nemergut ME, Warner D. Preparation of modern anesthesia workstations for malignant hyperthermia–susceptible patients: A review of past and present practice. Anesthesiology 2011; 114:205–212. PubMed
Other References
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.