Copy link
Spinal Cord Injury
Last updated: 05/24/2023
Key Point
- Spinal cord injury (SCI) at higher levels (i.e., upper thoracic and cervical) may culminate in neurogenic and hypovolemic shock.
- Succinylcholine is contraindicated 48 hours after a severe SCI due to concerns for hyperkalemia.
- Autonomic dysreflexia occurs most often in injuries at or above the T6 level and in chronic injury settings.
- Patients should be monitored closely for symptoms of autonomic dysreflexia, specifically with respect to blood pressure.
Introduction
- A spinal cord injury (SCI) is a medical emergency in which damage to the spinal cord or cauda equina results in potentially devastating motor, sensory or autonomic dysfunction.
- According to the National Spinal Cord Injury Statistical Center, the majority of SCIs result from trauma (tSCI), with motor vehicle collisions being the leading cause (37.6%), followed closely by falls (31.5%). Violence, particularly gunshot wounds, and sports injuries are other common causes.
- It is estimated that there are 18,000 new tSCI cases in the United States each year with 79% of cases occurring in men and an average age of 43 years old.1
Classification of Spinal Injury
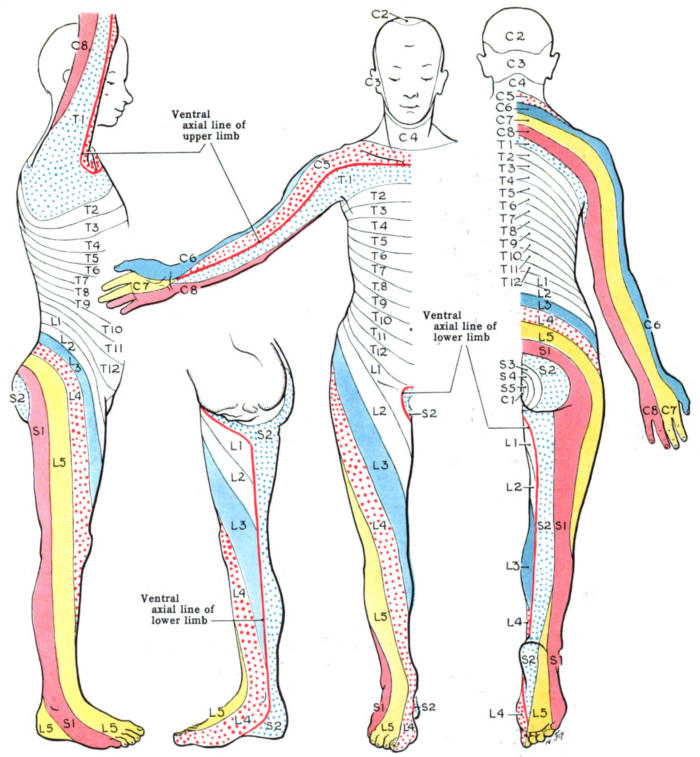
- SCI can present as impairments of sensory and motor signal transmission as well as autonomic dysfunction. Deficits along myotomal and dermatomal patterns are utilized to localize insults.
- The American Spinal Injury Association (ASIA) has developed a standardized exam to categorize SCI.2 The International Standards for Neurological Classification of SCI worksheet can be accessed here.
- Exams are performed separately for each side.
- The sensory exam determines the most caudal dermatome (Figure 1) that is intact to light touch and pinprick. Grading is on a 2-point scale (0 – absent, 1 – decreased or increased sensation, 2 – intact).
- The motor exam finds the most caudal myotome (Table 1) with a 3-point or greater score on the 5-point grading scale: (0 – total paralysis, 1 – palpable or visible contraction, 2 – movement without gravity, 3 – movement against gravity, 4 – movement with resistance, 5 – movement with full resistance).
- If no myotome can be assessed, the motor level is presumed to be equal to the sensory level.
- The neurological level of injury (NLI) is determined by the most caudal region with intact sensory (2/2) and motor (3/5) exams. The injury is then classified from A (no motor, no sensory, no sacral sparing) to E (normal motor and sensory function).
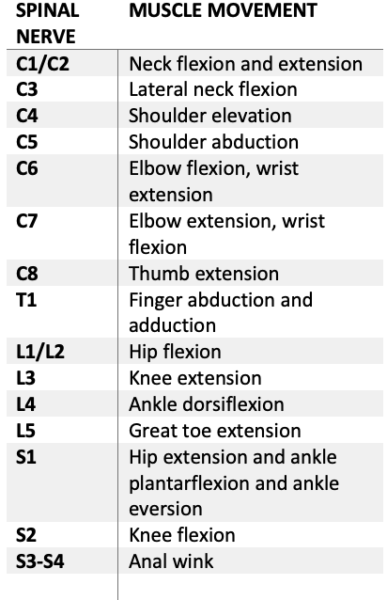
Table 1. Myotomes
Pathophysiology of SCI
- The primary injury phase is defined as the immediate aftermath of the insult consisting of either impact and persistent compression, impact and transient compression, distraction, laceration or transection.3
- Damage to the spinal cord may involve neural parenchyma, axonal pathways, glial membranes and adjacent vasculature, and may be associated with disruption of vertebral structures and ligaments.3
- Secondary injury is triggered by the primary injury and can be categorized into three phases: acute, subacute, and chronic injury (Figure 2). In the immediate aftermath, disruption of the vasculature and cell death can result in ischemia and localized edema.3
- The acute phase is characterized by progressive edema, ionic imbalances, cytokine upregulation, and elevated concentrations of oxygen species, as well as cellular infiltration of neutrophils, monocytes, microglial cells, astrocytes, and T cells.3
- Neuronal excitotoxicity resulting from intracellular calcium accumulation and dysregulated glutamate release may lead to disruption of nuclear acids, proteins, and phospholipids, ultimately leading to neuronal dysfunction.3
- Subacute secondary injury phase is characterized by neuronal apoptosis, axonal demyelination, Wallerian degeneration, and glial scar formation (Figure 2).3
- Chronic injury phase is characterized by the formation of cystic cavity, axonal dieback, and maturation of glial scar (Figure 2).3
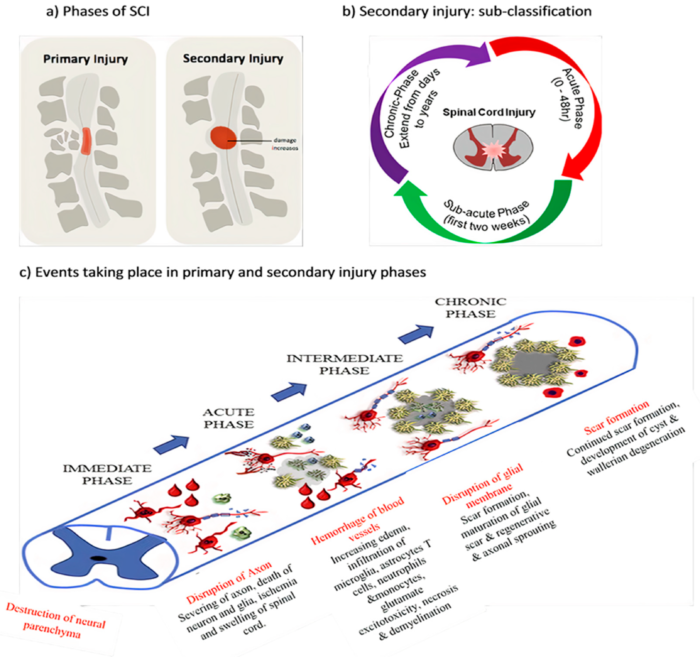
Figure 2. Phases of SCI. Source: Anjum A, et al. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 2020; 21(20):7533.2 CC BY.
Cardiovascular Effects of Acute SCI
- Spinal shock is common immediately after a SCI and is characterized by loss of spinal cord function caudal to the level of injury with flaccid paralysis, loss of sensation, absent bowel and bladder function, and absent reflexes. This can last days to weeks after SCI.5
- Neurogenic shock is a part of the spinal shock syndrome. It is common in complete cervical SCIs and involves loss of sympathetic tone leading to severe hypotension, bradycardia, hypothermia, and arrhythmias.4,5
- Hypotension can result in spinal cord hypoperfusion and exacerbate secondary SCI. Current guidelines recommend maintaining mean arterial pressures (MAP) greater than 85-90 mm Hg for at least 5-7 days after an acute SCI. In addition to fluids, vasopressors such as norepinephrine, epinephrine, dopamine, and phenylephrine are commonly used to maintain target MAPs. There is a paucity of convincing data to recommend one agent over others.4
Respiratory Effects of Acute SCI
- The respiratory effects of an acute SCI depend on the level and completeness of injury and the patient’s preexisting respiratory status.5,6 High cervical SCI affect respiration to a much greater extent than lower SCI.
- Above C3: patients are likely to be fully ventilator dependent due to diaphragmatic paralysis;
- C3-C4: diaphragmatic function is likely to be severely impaired, but some patients may be able to have some time off the ventilator while awake and sedated;
- C5: patients often need ventilator support initially but may be able to be weaned from the ventilator long-term;
- C6-C8: patients are able to breathe independently. Pectoral muscles may be used to support cough;
- T1-T4: intercostal muscles remain intact to support inspiration and forced expiration. Inability to use abdominal muscles weakens coughing;
- T4-T12: there is a gradual improvement in the strength of abdominal muscles;
- Below T12: respiratory function is essentially normal.
Autonomic Dysreflexia
- The term autonomic dysreflexia (AD) is used to describe an exaggerated sympathetic response to stimuli below the SCI, resulting in profound vasoconstriction and hypertension.7,8
- AD most often presents in injuries at or above T6 level.8
- AD is most common in chronic SCI patients, but may also occur in acute SCI.7
- Signs and symptoms of AD include7,8
- acute hypertension (sometimes with systolic blood pressure greater than 200 mmHg);
- arrhythmias (bradycardia, tachycardia, heart block, sinus arrest, etc.);
- vasoconstriction below spinal lesion and vasodilation above lesion;
- facial flushing;
- headache and nasal congestion (in awake patients);
- myocardial infarction, left heart failure; and
- intracranial hemorrhage, and seizures.
- Inciting factors include bladder distension and even pain in areas of lost sensation. General, spinal, and regional anesthesia have been shown to prevent AD in response to surgery.7
- History of AD should be elicited from the patient including triggers, symptoms, and management.
- Intra-arterial blood pressure monitoring in those with severe AD is important for the early detection and management of critical hypertension.7
- Treatment of intraoperative AD includes7
- Remove inciting stimulus: pause surgery, remove endoscope, evacuate bladder, etc.
- Deepen anesthetic: administer propofol bolus or increase volatile agent
- Adminster 100% oxygen
- Raise the head of the bed for orthostatic drop in blood pressure
- Adminster vasodilator: nicardipine 0.2-0.5 mg intravenous bolus and infusion, nitroglycerin or nitroprusside infusion
- Treat arrhythmias with beta blockers or anticholinergics
- Place intra-arterial catheter if AD does not resolve quickly.
Anesthetic Management of Acute SCI
Preoperative
- A focused airway assessment must include an assessment of the mouth opening, dentition, maxillofacial injuries, head trauma, and patient cooperation. The patient often presents with the cervical spine immobilized in a halo or collar. The cervical spine should be assumed to be unstable and adequate precautions taken (see below).
- Higher-level cervical SCI can compromise the function of the diaphragm and accessory muscles leading to hypoxia and hypoventilation, which may require intubation for invasive ventilation.4,5
- Trauma patients may present with other forms of shock, including hemorrhagic and obstructive shock.4
- Patient optimization with fluid resuscitation should be implemented prior to induction of anesthesia.5
- Patients with lower-level SCI may initially retain their respiratory function but may develop increased secretions leading to mucous plugging, aspiration pneumonia and respiratory failure.4,5
- Succinylcholine is contraindicated 48 hours after SCI due to concerns for hyperkalemia.5
- Nondepolarizing agents and/or remifentanil may be used for akinesis.5
- Anticholinergics should be used in thoracic and cervical SCI given concern for severe bradycardia in patients when neurogenic shock or bradycardia at baseline.4
- While the administration of steroids had been previously recommended for the management of acute SCI, more recent meta-analyses have failed to show statistically significant improvement in short-term or long-term neurologic outcomes. Instead, steroid administration has been associated with an increased risk of infection, pneumonia, and hyperglycemia.9,10
Intraoperative
- Manipulation of the cervical spine should be minimized during all phases of care. Manual in-line stabilization should be used during mask ventilation and intubation to prevent further SCI.5
- Airway management in patients with cervical SCI may prove challenging due to the need to minimize neck extension, potential associated facial injuries, airway edema, or the presence of cervical spine stabilization devices (i.e., halo or hard collar) that impede access to the airway
- Patients presenting with acute SCI for emergency surgery are at higher risk for aspiration, especially those with respiratory compromise.5 Rapid sequence induction and intubation is recommended to minimize the risk of aspiration; however, the use of cricoid pressure should be avoided due to the risk of worsening distraction and translation of the unstable cervical spine.5 Moreover, the efficacy of cricoid pressure in preventing aspiration is not well established.
- Awake intubation using a fiberoptic scope should be considered in cooperative patients in whom airway management (mask ventilation and/or endotracheal intubation) is anticipated to be difficult, with the benefit of maintaining spontaneous ventilation until the airway is secure and minimizing cervical spine manipulation.5 However, provider expertise is vital for success.
- Compared to direct laryngoscopy, the use of video laryngoscopy for patients who require cervical spine immobilization (either by MILS or with other immobilization devices) has been shown to improve the view of the glottis, reduce the degree of cervical spine motion and increase first-pass success rate.11
- In addition to the standard American Society of Anesthesiologists monitors, continuous hemodynamic monitoring with an arterial catheter should be considered, especially with high thoracic and cervical SCIs.
- SCI often leads to vasodilation and hypothermia below the level of injury; active warming is essential.4,5
- As mentioned previously, MAPs should be maintained above 85-90 mmHg to maintain adequate spinal cord perfusion.5
- Vasoactive medications with alpha and beta-1 receptor activity (e.g., norepinephrine) are usually required with SCIs at T1 to T4 who have lost cardiac sympathetic innervations.5
Anesthetic Management of Chronic SCI
Preoperative
- Patients with SCI are at a 3-10 times increased risk of coronary artery disease and associated cardiac complications.6 Preoperative pharmacologic stress tests to ascertain cardiac status can be valuable.
- These patients are more likely to be anemic, hypotensive, and hypovolemic at baseline. Hyponatremia related to disruption of the renin-angiotensin-aldosterone system pathway may be present.7 Preoperative fluid resuscitation to correct baseline hypovolemia and electrolyte disturbances should be considered.
- Care must be taken when premedicating with sedatives given the risk of respiratory depression.7
- Respiratory compromise is more likely to occur in higher level and more complete SCI. Patients often develop reduced lung volumes which increase their risk of desaturating on induction, and a partially impaired cough can place patients at greater risk of aspiration pneumonia.7
- Succinylcholine is contradicted in patients with SCI due to the risk of hyperkalemia; nondepolarizing agents or remifentanil should be used as alternatives.7
- While regional and spinal anesthesia can be used as appropriate, epidural anesthesia has been found to be unreliable, especially with deformities.7
Intraoperative
- Patients can experience thermoregulatory dysfunction resulting in hyperthermia or hypothermia.7
- Patients are also more susceptible to vasodilatory and myocardial depressant effects which can result in hypotension.7
- Adequate depth of anesthesia must be achieved prior to airway manipulation and tracheal suctioning, as increased vagal tone can lead to bradycardia.7 Vagolytic agents can be used in patients with a history of increased vagal tone.7
Postoperative
- Patients may benefit from assisted mechanical ventilatory support.7
- Longer recovery stays may be required to ensure hemodynamic, airway, and temperature stabilization.7
- These patients often suffer from chronic pain and may be maintained on significant preoperative opioids with resultant tolerance.7 Multimodal analgesia should be considered.7
References
- The National Spinal Cord Injury Statistical Center. Accessed on April 19, 2023. Link
- International Standards for Neurological Classification of SCI (ISNCSCI) Worksheet. American Spinal Injury Association. Accessed on April 21, 2023. . Link
- Anjum A, Yazid MD, Daud MF, et al. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 2020; 21(20):7533. PubMed
- Rogers WK, Todd M. Acute spinal cord injury. Best Pract Res Clin Anaesthesiol. 2016;30(1): 27-39. PubMed
- Mathews L, Kla K. Anesthesia for adults with acute spinal cord injury. In: Pasternak JJ (Ed). UpToDate. 2022. Accessed November 25th, 2022. Link
- Berlowitz DJ, Wadsworth B, Ross J. Respiratory problems and management in people with spinal cord injury. Breathe (Shelf). 2016:12(4): 328-40. PubMed
- Mathews L, Kla K. Anesthesia for adults with chronic spinal cord injury. In: Pasternak JJ (Ed). UpToDate. 2022. Accessed November 25th, 2022. Link
- Allen KJ, Leslie SW. Autonomic dysreflexia. In: StatPearls [Internet]. Accessed April 21, 2023. Link
- Sultan I, Lamba N, Liew A, et al. The safety and efficacy of steroid treatment for acute spinal cord injury: A Systematic Review and meta-analysis. Heliyon. 2020;6(2):e03414. PubMed
- Canseco JA, Karamian BA, Bowles DR, et al. Updated Review: The steroid controversy for management of spinal cord injury. World Neurosurgery. 2021;150: 1-8. PubMed
- Robitaille A, Williams SR, Tremblay MH, et al. Cervical spine motion during tracheal intubation with manual in-line stabilization: direct laryngoscopy versus GlideScope videolaryngoscopy. Anesth Analg. 2008;106(3):935-41. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.