Copy link
Regional Anesthesia in Patients Receiving Antithrombotic or Thrombolytic Therapy: Part 1
Last updated: 04/06/2023
Key Points
- The American Society of Regional Anesthesia and Pain Medicine (ASRA) recommends against performance of spinal or epidural anesthetics in patients who have received fibrinolytic and thrombolytic drugs (grade 1A).
- Heparin administration after catheter removal can occur immediately after neuraxial blockade or catheter removal. This recommendation is updated in the erratum from the previous 1-hr time interval found in the original article.1,4
- Due to the risk for heparin-induced thrombocytopenia (HIT), patients receiving unfractionated heparin (UFH) or low molecular weight heparin (LMWH) for more than 4 days should have a platelet count assessed prior to neuraxial block or catheter removal (grade 1C).
Introduction
- In 2018, ASRA partnered with National Partnership for Maternal Safety (NPMS) and the Society for Obstetric Anesthesia and Perinatology (SOAP) to create the 4th edition of the ASRA guidelines on regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy.1,2
- Notable updates from the 2015 guidelines include new and adjusted recommendations for newer medications, including herbal supplements, and the routine use of physical exam findings for signs of coagulopathy.1,3
- It is important to note that these guidelines do not define the standard of care and are not intended to replace clinical judgment. The phrase “ASRA recommends” is used for strong recommendations (grades 1A, 1B, and 1C), and “ASRA suggests” is used for weaker recommendations (grades 2A, 2B, and 2C).1
- ASRA suggests that these guidelines may be applied to parturients (grade 2C). However, in circumstances involving select high-risk parturients receiving venous thromboembolism (VTE) prophylaxis and requiring urgent interventions for maternal or fetal indications, exceptions/modifications of guidelines may be appropriate (grade 2C).
- This summary will focus on the recommendations for patients on fibrinolytic/thrombolytics and heparin.
Fibrinolytics and Thrombolytics
- Patients receiving fibrinolytic or thrombolytic medications are at risk of serious hemorrhagic events such as spinal hematomas, particularly those who have undergone an invasive procedure.1
- ASRA recommends against performance of spinal or epidural anesthetics in patients who have received fibrinolytic and thrombolytic drugs (grade 1A).1 While there is no clear data on how long to wait, ASRA suggests waiting for at least 48 hours and documenting normalization of clotting and fibrinogen levels (grade 2C).1
- There is no definitive recommendation for the removal of neuraxial catheters in patients who unexpectedly receive fibrinolytics and thrombolytics during a neuraxial catheter infusion. Frequent neurological checks (every 2 hours) should be performed for an appropriate interval. ASRA suggests measuring fibrinogen levels to evaluate the presence of residual thrombolytic effect and appropriate timing of removal of epidural catheters (grade 2C).1 Fibrinogen is one of the last clotting factors to recover.
Intravenous and Subcutaneous Unfractionated Heparin
- Patients receiving intravenous (IV) or subcutaneous (SC) unfractionated heparin (UFH) for more than 4 days should have a platelet count assessed prior to neuraxial block or catheter removal as heparin-induced thrombocytopenia (HIT) may occur (grade 1C).1
Intravenous Heparin
- The heparin infusion should be discontinued for 4-6 hours and normal coagulation status verified prior to neuraxial blockade (grade 1A).1
- Heparin administration should be delayed for 1 hour after needle placement (grade 1A).1
- Indwelling neuraxial catheters should be removed 4-6 hours after the last IV heparin dose (and after assessment of the patient’s coagulation status); reheparinize 1 hour after catheter removal (grade 1A).1
- A dilute concentration of the local anesthetic should be used and the patient monitored postoperatively for early detection of motor blockade (grade 1A).1
- There is no data to support the mandatory cancellation of a case with a bloody or difficult neuraxial placement (grade 1A).1
Subcutaneous Heparin
- Preoperative low-dose SC heparin (5000 U BID or TID): ASRA suggests performing neuraxial block 4-6 hours after heparin administration, or coagulation status be assessed (grade 2C).1
- Preoperative higher dose SC UFH (7500-10,000 U BID or a daily dose of < 20,000 U): ASRA suggests neuraxial block 12 hours after SC heparin administration and assessment of coagulation status (grade 2C).1
- Preoperative therapeutic SC UFH (>10,000 U dose or > 20,000 U total daily dose): ASRA suggests neuraxial block 24 hours after SC heparin administration and assessment of coagulation status (grade 2C).1
- Postoperative low-dose SC heparin: ASRA suggests catheter removal 4-6 hours after heparin administration, and subsequent heparin administration may occur immediately after neuraxial blockade or catheter removal (grade 2C).1
- Postoperative “higher-dose” SC heparin (>5000u or >15,000 U daily): The safety of indwelling neuraxial catheters has not been established in this scenario. Risks and benefits should be assessed on a case-by-case basis, and techniques to facilitate the detection of new or progressive neurologic deficits (grade 2C).
Low-Molecular-Weight Heparin
- Low-molecular-weight heparins are used for the prophylaxis and treatment of arterial and VTE. Antifactor Xa levels peak 3-5 hours after administration, and the elimination of LMWH is 3-6 hours after SC injection in a patient with normal renal function.1
- There are no adequate trials comparing the efficacy and safety of one LMWH to another, and it is not possible to recommend one specific LMWH over another. Data suggest that the rate of spinal hematoma is similar among LMWH preparations.5
- Antiplatelet or oral anticoagulation medications coadministered with LMWH increase the risk of spinal hematomas. When there is an indwelling neuraxial catheter, ASRA recommends against the concomitant administration of antiplatelet and oral anticoagulation medications along with LMWH, regardless of the LMWH dosing regimen.1
- Patients receiving LMWH for more than 4 days should have a platelet count assessed prior to neuraxial block or catheter removal (grade 1C).
- ASRA recommends against the routine monitoring of antifactor Xa levels (grade 1A).1
Preoperative LMWH Dosing
- Preoperative prophylactic LMWH dosing: Needle placement should occur at least 12 hours after LMWH dose (grade 1A).1
- Preoperative higher therapeutic LMWH dosing: Needle placement should occur at least 24 hours after LMWH dose (grade 1A).1
Postoperative LMWH Dosing
- Postoperative BID prophylactic dosing: This dosing regimen is associated with an increased risk of spinal hematoma. ASRA recommends that the first LMWH dose should be administered the following day and no earlier than 12 hours after needle placement. Indwelling catheters should be removed prior to the initiation of LMWH prophylaxis. LMWH administration should be delayed for 4 hours after catheter removal.1
- Single daily prophylactic dosing: ASRA recommends that the first postoperative LMWH should be administered at least 12 hours after needle placement. The second dose should occur no sooner than 24 hours after the first dose. Indwelling catheters may be maintained. The catheter should be removed 12 hours after the last dose, and subsequent dosing should occur at least 4 hours after catheter removal (grade 1C).
- Single or BID therapeutic dosing: Therapeutic dose LMWH may be resumed 24 hours after non-high-bleeding-risk surgery and 48 hours after high-bleeding-risk surgery. Indwelling catheters should be removed 4 hours prior to the first postoperative dose and at least 24 hours after catheter placement, whichever is greater (grade 1C).1
See also: Regional Anesthesia in Patients Receiving Antithrombotic or Thrombolytic Therapy: Part 2
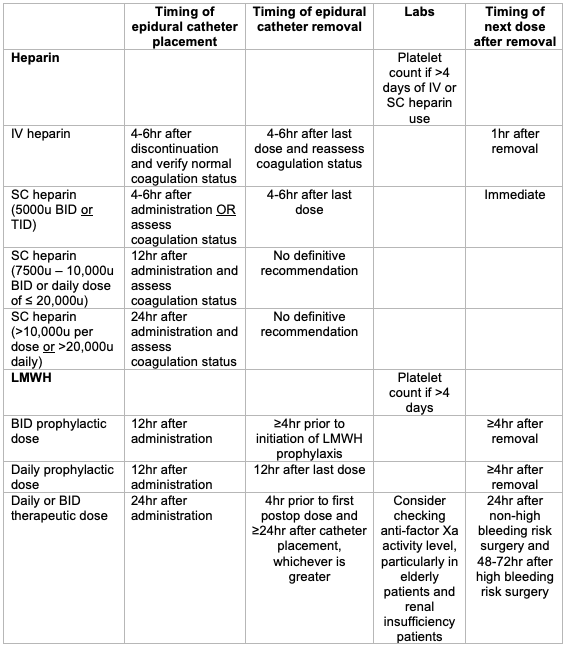
Table 1. Summary of ASRA recommendations for epidural catheter placement and removal in patients on heparin.
References
- Horlocker TT, Vandermeuelen E, Kopp SL, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (Fourth edition) Reg Anesth Pain Med. 2018;43(3):263-309. PubMed
- Leffert L, Butwick A, Carvalho B, et al. The Society for Obstetric Anesthesia and Perinatology consensus statement on the anesthetic management of pregnant and postpartum women receiving thromboprophylaxis or higher dose anticoagulants. Anesth Analg. 2018;126(3):928-944. PubMed
- Wickboldt A, Guirguis M. ASRA Pain Medicine News. ASRA Pain Medicine. Published August 27, 2019. Accessed October 30, 2022. Link
- Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (Fourth edition): Erratum. Reg Anesth Pain Med. 2018;43(5):566. PubMed
- Tryba M. European practice guidelines: thromboembolism prophylaxis and regional anesthesia. Reg Anesth Pain Med. 1998;23(6 Suppl 2):178-182. PubMed
Other References
- Bauer ME, Arendt K, Beilin Y, et al. The Society for Obstetric Anesthesia and Perinatology Interdisciplinary Consensus Statement on Neuraxial Procedures in Obstetric Patients With Thrombocytopenia. Anesth Analg . 2021 Jun 1;132(6):1531-1544. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.