Copy link
Spinal Anesthesia in Adults: Pharmacology, Technique, and Side Effects
Last updated: 12/21/2023
Key Points
- Spinal anesthesia is achieved through the introduction of local anesthetic (LA) to the lumbar cerebrospinal fluid (CSF), which diffuses down a concentration gradient across axonal cell membranes, binding to voltage-gated sodium channels on the cytoplasmic surface, triggering autonomic, sensory, and motor blockade.
- Anatomical landmarks are generally reliable in identifying vertebral levels, with point-of-care neuraxial ultrasonography being helpful in some cases.
- The spread of LA can be affected by the addition of adjuvants, level and duration of blockade, volume and concentration, and patient positioning.
Pharmacology of Local Anesthetics
- Bupivacaine, ropivacaine, mepivacaine, and 2-chloroprocaine are commonly used LA for spinal anesthesia.
- Pharmacokinetics of the LA depends on local blood flow, degree of protein binding, lipophilicity, LA type, and adjuvants.1
- Once the local anesthetic is introduced to the lumbar CSF, the nonionized molecule diffuses down a concentration gradient across the axonal cell membranes, binding to voltage-gated sodium channels on the cytoplasmic surface triggering autonomic, sensory, and motor blockade several dermatomal levels above and below the site of injection.1
- Due to the lack of dura, the LA solution is rapidly taken up by nerve roots and rootlets bathed in CSF and is also absorbed across the pia mater into the spinal cord, primarily concentrating in the posterior and lateral corticospinal and spinothalamic tracts.1
- Metabolism of LA does not occur in the CSF and instead depends on local blood flow, degree of protein binding, lipophilicity, LA type, and adjuvants.1
- Factors determining the spread of LA solution are listed in Table 1.
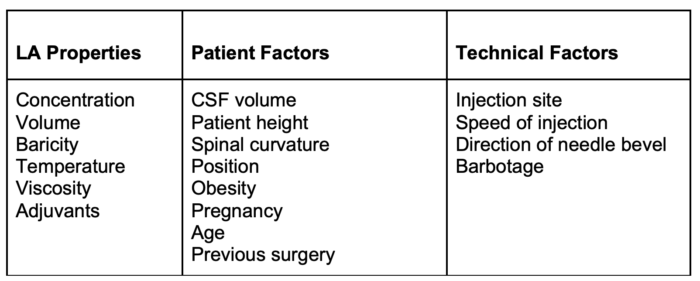
Table 1. Factors determining the spread of LA after spinal administration
Adjuvants
Opioids
- Intrathecal and epidural opioids are commonly administered alongside local anesthetic agents to achieve synergistic analgesia and anesthesia.
- Long-acting opioids (preservative-free morphine, or less commonly, diacetylmorphine) provide long-lasting analgesia due to their persistence in the CSF but can cause side effects such as sedation, pruritus, nausea and vomiting, urinary retention, and hypoventilation.1
- Short-acting opioids (such as fentanyl and sufentanil) are more lipophilic and can cause signs of systemic absorption within minutes of injection. They are commonly added to patient-controlled epidural anesthesia infusions for labor or postoperative pain.1
Epinephrine
- Preservative-free epinephrine is commonly added to both intrathecal and epidural local anesthetic solutions at a concentration of 5mcg/ml.
- Epinephrine both potentiates and speeds the onset of neuraxial blockade, prolongs the duration of short-acting LA, and acts as a marker of inadvertent intravascular injection.
- The addition of epinephrine can be associated with an increased risk of some neurologic complications.1,2
- Additionally, there is a risk of drug error when diluting the solution.
Alpha-2 Agonists
- Clonidine and dexmedetomidine may be added to both intrathecal and epidural LA solutions, potentiating and prolonging neuraxial blockade via α2 agonism at the spinal cord and dorsal root ganglia.1
- Sedation and lingering hypotension are the most common side effects, and prolonged hypotension in some cases is an important limitation, particularly with the less α2-selective clonidine.1
Level and Duration
- Level and duration are primarily determined by 1) characteristics of the LA solution (e.g., baricity, measure of the relative density of the LA compared with CSF, 2) contour of the spinal canal, and 3) patient position in the first few minutes after injection.1
- Hyperbaric LA solutions are denser than CSF and tend to follow gravity after injection. When using hyperbaric solutions, the operative side should be dependent.
- Isobaric solutions result in less spread than hyperbaric solutions but can exhibit slightly hyperbaric behavior because of their low temperature. These solutions are best suited for perineal or lower extremity surgeries.
- Hypobaric solutions are LA solutions diluted with sterile water or 1/2 normal saline, the latter of which causes less osmotic stress to neural tissues.3
- Spread can also be affected by the addition of vasoconstrictors (e.g., 0.1 – 0.2 mg of epinephrine, or 0.2 to 0.5 cc of 1:1000 epinephrine). Epinephrine may have the additional benefit of some alpha-2-related analgesia. Tetracaine receives the most benefit from vasoconstriction.1
Technique
Setup and Positioning
- Appropriate monitoring should include standard American Society of Anesthesiologists monitors, including noninvasive blood pressure, electrocardiography, pulse oximetry, and capnography.
- The seated position with an exaggerated kyphosis can help identify the midline and optimize the vertebral spaces, in turn offering the best first-pass success rates, although certain clinical requirements may necessitate lateral positioning (e.g., patients with hip fractures).1
- A pillow under the patient’s knees or placing the patient in the lateral position can help optimize the placement of the spinal when lumbar lordosis is nonoptimal.
- Anatomical landmarks are generally reliable in identifying vertebral levels.
- Point-of-care neuraxial ultrasonography is helpful in identifying the ideal level, midline, and depth to the epidural space in some cases.1
Equipment and Needles
- Bactericidal skin preparation solution, sterile drape, hypodermic needles for skin infiltration.
- LA agent, introducer needle, and spinal needle.
- Commonly used spinal needles:
- Pencil-point needles with noncutting bevels and a solid tip: e.g. Sprotte, Whitacre.2
- Needles with cutting bevels: e.g. Quincke, Pitkin.2
- Rounded point needle with noncutting bevel: Greene.2
- Resuscitation equipment to secure the airway, provide ventilation, and hemodynamic support must be available.
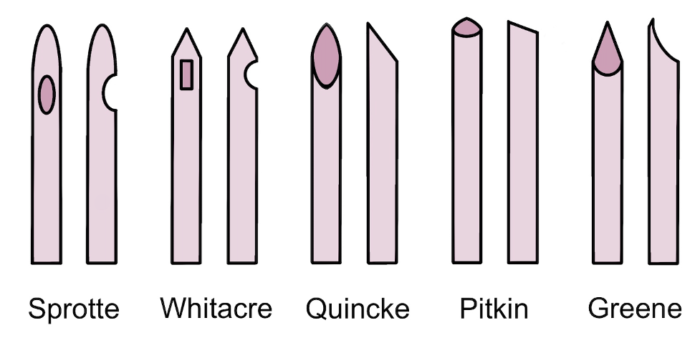
Figure 1. Common spinal needle shapes. Own work, 2023.
Technique
- A line drawn from the top of the iliac crest is known as “Tuffier’s” line. This line generally crosses the body of L4 or the L4-L5 interspace. The conus medullaris, the terminal segment of the spinal cord, may extend down to L3 in about 2% of adults. Therefore, palpating the top of the iliac crests helps locate an ideal space for spinal needle entry.1,2
- The midline approach is typically considered the easiest, while the paramedian approach is well-suited to narrow interspaces or difficulty with flexion.1,2
- After placement of an introducer needle, a small gauge needle is used to enter the dura. Pencil-point needles are associated with a lower risk of postdural puncture headache (PDPH), as are thin gauges (frequently 25g or 27 gauge). Once CSF is flowing from the needle, the medication is injected, with or without mixing of withdrawn CSF in a technique known as barbotage. The needle is then withdrawn, and the patient is assisted into a flat position.1,2
Side Effects and Complications
- Hemodynamic changes are common after spinal anesthesia, with drops in systemic vascular resistanc and preload.1,2
- Hypotension can be treated with a modest reverse Trendelenburg position and hydration, with a mixed adrenergic agonist ephedrine as the first-line drug.1,4
- LA systemic toxicity can result in prolonged cardiac arrest due to complex effects on myocardial lipid metabolism (though this is unlikely given typical low doses used in single-shot neuraxial techniques).5
- PDPH risk can be reduced with pencil-point needles and by directing the opening towards the desired flow direction.
- High spinal anesthesia occurs when the spread of the LA affects spinal nerves above T4, and it can result in cardiovascular and/or respiratory compromise. LA flow even higher up into the cranium can result in loss of consciousness and seizures. This would require airway control and ventilation, intravenous fluids, and sympathomimetics.2
- Postspinal backache occurs in about 13% of patients and is postulated to occur secondary to a localized inflammatory response, often associated with a degree of muscle spasm. The backache is usually self-limited and treatment consists of conservative measures, including hot and cold compresses and oral analgesics.
- Transient neurologic symptoms (TNS) are characterized by unilateral or bilateral radiating pain in the buttocks and legs. Symptoms occur within 24 hours of intrathecal injection and usually resolve within 1 week. Incidence of TNS is highest with the use of lidocaine (~14%) and mepivacaine (wide variation, 0-30%), but occurrences of TNS have been described with all local anesthetics used for spinal anesthesia.1,2
- Other potential side effects include failed spinal, urinary retention, subdural injection, and hypoventilation.1,2
- Serious complications, including bleeding, infection, and nerve damage, are very rare.1,2
References
- Brull R, Macfarlane AJR, Chan VWS. Spinal, epidural, and caudal anesthesia. In: Miller’s Anesthesia. 9th ed. Elsevier.2020: 1413-49.
- Chin A, van Zundert A. Spinal anesthesia. In: Hadzic A, ed. Hadzic’s Textbook of Regional Anesthesia and Acute Pain Management. 2nd ed. McGraw-Hill Education. 2017.
- Pozek JPJ, Beausang D, Segna KG, Viscusi ER. Controlled-release local anesthetics. In: Hadzic A, ed. Hadzic’s Textbook of Regional Anesthesia and Acute Pain Management, 2e. McGraw-Hill Education; 2017.
- Kumar M, Bhandari S, Thakur A, et al. Investigating the effect of the 10° reverse Trendelenburg position on spinal block characteristics and hemodynamic parameters in lower limb surgeries. Cureus. 2022; 14(2):e22588. PubMed
- Dickerson DM, Apfelbaum JL. Local anesthetic systemic toxicity. Aesthet Surg J. 2014;34(7):1111-9. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.