Copy link
Pediatric Airway Anatomy
Last updated: 05/06/2024
Key Points
- The pediatric airway differs from the adult airway in several respects.
- A detailed understanding of the anatomical differences between the infant and the adult airway is paramount to the safe conduct of anesthesia in the pediatric patient.
- Unlike the adult patient, where the larynx is cylindrical, with the narrowest point being at the glottic opening, the pediatric airway is funnel-shaped, and the cricoid cartilage is the narrowest part.
Basics of Pediatric Airway Anatomy
Broadly speaking, the pediatric airway extends from the head and neck to the lower airways. Specific areas of importance for the anesthesiologist include the nose, mouth, pharynx, larynx, glottic aperture, subglottis, trachea, carina, and the main stem bronchi.1
Head and Neck
- The infant’s head is relatively large compared to its body due to rapid brain growth. The large occiput causes flexion of the head, predisposing the infant to upper airway obstruction in the supine position.
- Facial growth accelerates in late infancy as primary teeth and phonation begin to develop.
- Poor facial and mandibular development, usually associated with syndromes (e.g., Pierre Robin), usually results in difficulty with laryngoscopy.
Nose
- Infants are “preferential” nasal breathers because of the relatively large tongue impeding oral breathing. This resolves at about six months of age, allowing easy breathing through both orifices. The highly vascular Little’s area on the nasal septum is a common source of nose bleeds, especially associated with nasotracheal intubations.
Mouth
- The relatively large tongue causes airway obstruction at rest. The genioglossus muscle protrudes the tongue and maintains airway patency. Decreased muscle tone by anesthetics and sedatives results in upper airway obstruction unless steps are taken to alleviate it (jaw thrust, chin lift, oropharyngeal or nasopharyngeal airways). A magnetic resonance imaging study in children 1-11 years of age suggested that the role of the tongue in causing airway obstruction is minor and that nasopharyngeal and epiglottic collapse were more important factors.2
Pharynx
- The pharynx is a muscular tubular structure that forms the common communication between the respiratory and digestive tracts. The soft palate and epiglottic tip divide the pharynx into a superior nasopharynx, a middle oropharynx, and an inferior laryngopharynx or hypopharynx.1,3
- Hypertrophy of the nasopharyngeal tonsils (adenoids) is a common cause of airway obstruction in infancy and early childhood.1
- The palatine tonsils (commonly called “tonsils”), the lingual tonsils, and the nasopharyngeal tonsils (adenoids) form a ring of lymphoid tissue called the Waldeyer’s ring.1 Enlargement of any of these lymphoid tissues can result in airway obstruction, commonly requiring surgical removal.
- The laryngopharynx (hypopharynx) extends from the epiglottic tip to the lower border of the cricoid cartilage. The pyriform fossae lie on either side of the laryngopharynx and is a common site for foreign body impaction.
Larynx
- The larynx at birth is at the C3-4 vertebral level and descends to the C5 level in adolescence (Figure 1).
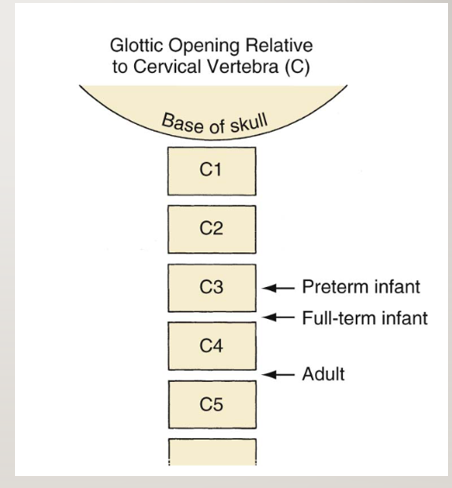
Figure 1. Relative locations of the larynx in a preterm infant, full-term infant, and adult. Reproduced with permission from Litman RS, et al. The pediatric airway. In: Coté CJ, Lerman J, Anderson J: A practice of anesthesia for infants and children. 5th edition. Philadelphia, PA. Elsevier, 2013. 237-276.
- Unlike older children, infants are able to feed and breathe at the same time because their larynx is higher, and during swallowing, the epiglottis slides up behind the soft palate, locking the larynx into the nasopharynx. This ability to feed and breathe at the same time is lost between 2 and 6 years of age as the larynx gradually descends into the adult location.
- The larynx is primarily a protective valve at the entrance to the respiratory tract. Its role in phonation was a much later evolutionary development. It consists of 10 cartilages (thyroid, cricoid, epiglottis, hyoid, and the paired arytenoid, corniculate, and cuneiform cartilages). These are connected by ligaments and move by the action of the laryngeal muscles.
- The thyroid is shield-like and consists of two laminae that meet in the midline.
- The superior laryngeal and recurrent laryngeal nerves, branches of the vagus nerve, innervate the larynx.3 The superior laryngeal nerve divides into external and internal branches. The external branch supplies the cricothyroid muscle. The internal branch provides sensory innervation to the interior of the larynx above the vocal cords. The recurrent laryngeal nerve provides motor innervation to all the other intrinsic laryngeal muscles (except the cricothyroid) and sensory innervation of the laryngeal mucosa below the vocal cords.
- The cricoid cartilage is shaped like a signet ring and is the only complete cartilage ring in the respiratory tract.
- The infant epiglottis is classically described as omega-shaped and narrow (Figure 2). It is floppy due to a lack of elastic cartilage, which makes it difficult to lift indirectly with a curved blade (i.e., Macintosh). Therefore, straight blades (i.e., Miller) are more effective at lifting the epiglottis during direct laryngoscopy in infants and small children.
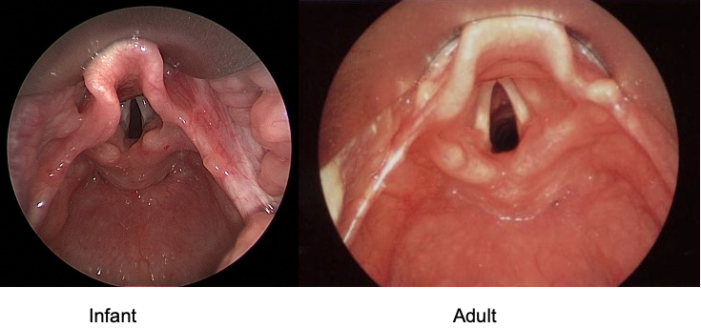
Figure 2. Rigid bronchoscopic views of the adult and infant larynxes.
- The anterior attachment of the vocal cords is slanted inferiorly (caudad) compared to the posterior attachment. This, coupled with the narrow anterior commissure, can occasionally impede endotracheal tube (ETT) advancement, especially during nasotracheal intubation.
- The neonatal cricothyroid membrane measures 2.6mm X 3.0mm compared to 10.4mm X 8.2mm in the adult.4 These extremely small dimensions make front-of-neck access extremely hazardous, especially in emergency situations, like cricothyroidotomy for a difficult airway.
Trachea and Bronchi
- The mean tracheal length at birth is 4 cm. From birth to adolescence, tracheal length doubles, tracheal diameter triples, and tracheal cross-sectional area increases six-fold.4 Meticulous ETT placement is therefore imperative to avoid mainstem intubation.
- The left and right main stem bronchi take off at similar angles, unlike in the adult, where the left main stem bronchus take off is more acute. Overenthusiastic ETT advancement will almost certainly result in a right mainstem intubation, likely related to the ETT bevel.
Respiratory Mechanics and Diaphragm
- The infant’s respiratory function is notoriously inefficient compared to older children and adults. Factors contributing to this include mechanically inefficient horizontal ribs projecting from the vertebral column, nonossified compliant ribs, and a very compliant rib cage with the propensity to lung collapse, decrease functional residual capacity (FRC), and rapid desaturation.
- Infants maintain their FRC despite these unfavorable conditions by contraction of the laryngeal adductor muscles prior to end-expiration. This limitation of expiratory flow prevents total lung collapse by maintaining positive end-expiratory pressure in the airway. This mechanism is called “laryngeal braking” or, in layman’s terms, “grunting.”
- The infant diaphragm is composed of only 10% of Type 1, slow-twitch, fatigue-resistant muscle fibers compared to the adult diaphragm. This predisposes them to rapid respiratory failure in conditions of increased work of breathing.
Other Airway Differences
Shape of the Pediatric Airway and Narrowest Portion
- Of the various differences between the pediatric and adult airways, none has caused the most disagreement and debate as the shape of the pediatric larynx and the location of the narrowest portion (Figure 3).
- To attempt to resolve this disagreement, Holzki et al. conducted a systematic review of relevant studies dating back to 1897 and concluded that in vivo studies did not consider the motion of the highly pliable laryngeal structures, the effects of anesthesia, and the phase of respiration when measurements were made.5 They concluded that in vitro studies using measuring rod or calipers were clearly superior and confirmed earlier findings that the infant larynx is in fact funnel-shaped and that the cricoid cartilage is the narrowest part (Figure 3). An ETT that passes the glottis in an adult will most likely advance unobstructed into the trachea. The same cannot be said for an infant or small child, as the ETT may still get stuck at the cricoid ring.
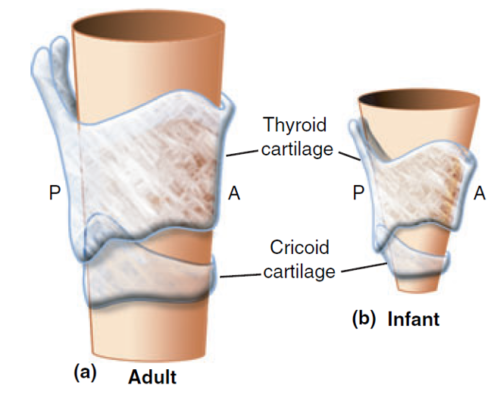
Figure 3. Shape of the adult and infant larynges. Reproduced with permission from Litman RS, et al. The pediatric airway. In: Coté CJ, Lerman J, Anderson J: A practice of anesthesia for infants and children. 5th edition. Philadelphia, PA. Elsevier, 2013. 237-276.
Airway Reactivity
- Compared to the adult airway, the pediatric airway is very reactive and more prone to laryngospasm and bronchospasm. Increased vagal tone, frequent upper respiratory infections, and use of inhalational anesthetic induction may be contributing factors.
- Reactive edema from a tight-fitting endotracheal tube can result in profound airway narrowing and postextubation stridor. Figure 4 shows the relative effects of a 1 mm ring of edema in a pediatric and an adult airway. Proper ETT selection and gentle airway instrumentation will minimize the risk of airway complications in infants and small children.
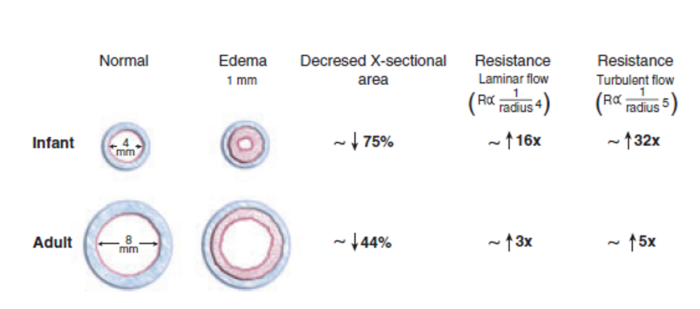
Figure 4. Relative effects of airway edema in infants and adults. Reproduced with permission from Litman RS, et al. The pediatric airway. In: Coté CJ, Lerman J, Anderson J: A practice of anesthesia for infants and children. 5th edition. Philadelphia, PA. Elsevier, 2013. 237-276.
References
- Adewale L. Anatomy and Assessment of the pediatric airway. Pediatr Anaesth. 2009;19 (Suppl. 1): 1-8. PubMed
- Arens R, McDonough JM, Corbin AM, et al. Linear dimensions of the upper airway structure during development: assessment by magnetic resonance imaging. Am J Respir Crit Care Med. 2002; 165: 117-22. PubMed
- Ellis H, Feldman S, Harrop-Griffiths W. The Respiratory Pathway. In: Anatomy for Anaesthetists: Blackwell Publishing, 2004: 3 - 68
- Monnier P. Applied surgical anatomy of the larynx and trachea. In: Pediatric airway surgery, New York, Springer, 2011:7 29.
- Holzki J, Brown KA, Carroll RG, Cote CJ. The anatomy of the pediatric airway: Has our knowledge changed in 120 years? A review of historic and recent investigations of the anatomy of the pediatric larynx. Pediatr Anaesth. 2018; 28: 12-22. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.