Copy link
Aneurysmal Subarachnoid Hemorrhage Part 1: Epidemiology, Pathophysiology, Diagnosis, Cardiac Complications
Last updated: 07/18/2023
Key Points
- A subarachnoid hemorrhage (SAH) is a neurologic emergency that commonly results from the rupture of a saccular aneurysm.
- Risk factors for SAH include aneurysm size and location, smoking, hypertension, family history, alcohol abuse, use of sympathomimetic drugs, etc.
- The classic clinical presentation is sudden onset, severe headache, often described as the “worst headache of my life.”
- A noncontrast computed tomography is the cornerstone for the diagnosis of SAH.
Epidemiology
- Stroke is the second leading cause of death. In the United States, the annual incidence of a new or recurring stroke is 795,000, of which 87% are ischemic, 10% are intracranial hemorrhages (ICH), and 3% are SAH.1 Globally, 62% are ischemic, 28% are ICH, and 10% are SAH.
- The two main types of brain hemorrhage are ICH and SAH.
- ICH refers to bleeding directly into the brain parenchyma. The most common causes are hypertension, trauma, bleeding diathesis, illicit drug use, vascular malformations, etc.
- SAH refers to bleeding into the cerebrospinal fluid within the subarachnoid space that surrounds the brain. Most cases of SAH are aneurysmal in origin (aSAH) and result from rupture of arterial aneurysms at the base of the brain. The other main cause of SAH is vascular malformations near the pial surface.
- This summary will focus on aSAH.
- The global incidence of aSAH is 2 to 16 per 100,000. The incidence varies by ethnicity and geography.
- The lowest incidence of SAH is in China, followed by Central and South America, United States, while the highest incidence is in Finland and Japan.2
- Women are more likely to be affected than men by a factor of 1.24.3
- A higher incidence of SAH is seen among Black and Hispanic patients.
- Most aneurysms occur between 40-60 years of age, with a mean age of rupture between 50 and 55 years.
- Aneurysms are typically located at branching points within the circle of Willis, and the common sites include the anterior cerebral artery, anterior communicating artery, posterior communicating artery, middle cerebral artery, and basilar artery (Figure 1).
- Risk factors for SAH include4:
- Aneurysm size and location: larger aneurysms and posterior circulation aneurysms at an increased risk for rupture
- Smoking: most important preventable risk factor for SAH
- Hypertension: major risk factor
- Genetic risk and family history of SAH
- Prior history of aSAH
- Moderate to heavy alcohol abuse
- Use of sympathomimetic drugs: methamphetamine and cocaine abuse
- Estrogen deficiency
- Antithrombotic therapy, etc.
- SAH is associated with an increased risk of mortality:
- About 10-15% of patients die before hospitalization, and 25% die within the first 48 hours.3
- Initial hemorrhage is usually the primary cause of death, followed by rebleeding.3
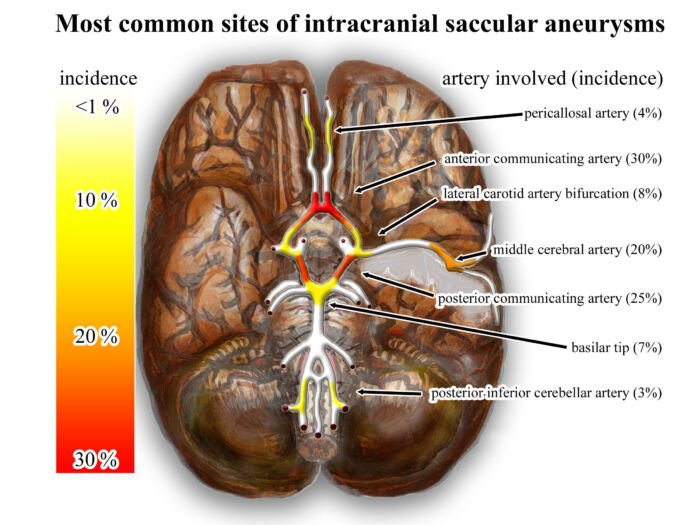
Figure 1. Heat map indicating the common location for cerebral aneurysms. Source: Wikimedia Commons. Link.
Pathophysiology
- Most aSAH result from the rupture of a saccular (“berry”) aneurysm, which is a thin-walled outpouching of the arterial wall with thin or absent tunica media and absent or fragmented internal elastic lamina.5
- Most cases occur without an identifiable trigger, but some cases may be preceded by an acute trigger, such as physical exertion.4
- Rupture of the aneurysm rapidly releases blood into the intracranial cisterns and subarachnoid space within seconds.
- The SAH may result in loss of consciousness secondary to global ischemia from raised intracranial pressure (ICP), decreased cerebral perfusion pressures, and cerebral blood flow.
- The force of the arterial hemorrhage can also decompress the hemorrhage into the intraparenchymal space causing an associated intraparenchymal hemorrhage (IPH), or into the ventricular space causing intraventricular hemorrhage (IVH).
- Direct pressure from acutely expanding associated IPH causes local compression of surrounding brain tissue and mechanical injury by elevated ICP.
- Hydrocephalus caused by IVH and damage to the arachnoid villi prevents cerebral spinal fluid (CSF) resorption.
- Brain herniation can occur both from hydrocephalus and expanding IPH.
- Secondary physiological/cellular pathways are triggered by the subarachnoid hemorrhage, intraventricular hemorrhage and hematoma and its metabolized blood products.
Clinical Presentation & Diagnosis
- The classic clinical presentation of aSAH includes a sudden onset, severe headache (or thunderclap headache), often described as the “worst headache of my life.” Half of the patients lose consciousness. Other symptoms include nausea/vomiting, nuchal rigidity, or photophobia.5
- Physical examination often reveals hypertension and signs of meningismus.
- Other neurological findings depend on the location of SAH.6
- Third nerve palsy → usually posterior communicating aneurysm
- Sixth nerve palsy → elevated ICP
- Hemiparesis and aphasia → middle cerebral artery aneurysm
- Retinal or preretinal hemorrhages → elevated ICP
- Bilateral leg weakness and abulia → anterior communicating artery aneurysm
- Brainstem signs → basilar artery aneurysm
- Several clinical grading systems are used. The Hunt-Hess score is commonly used as a predictor of prognosis/outcome, with a higher score correlating to a lower survival rate. When originally proposed, the scale ranged from a score of 1 to 5.5
- Grade 0: Unruptured aneurysm
- Grade I: Asymptomatic or minimal headache and slight nuchal rigidity
- Grade Ia: Fixed neurological deficit without other signs of SAH
- Grade II: Moderate to severe headache, nuchal rigidity, no deficit other than cranial nerve palsy
- Grade III: Drowsiness, confusion, or mild focal deficit
- Grade IV: Stupor, moderate to severe hemiparesis, early decerebrate rigidity, and vegetative state
- Grade V: Deep coma, decerebrate rigidity, moribund appearance
- The Fisher scale is an index of vasospasm risk based on the computed tomography (CT) appearance of the SAH (amount and distribution of SAH). The modified Fisher scale (also known as the Claassen scale) is a similar index of the risk of delayed cerebral ischemia from vasospasm.5,6
- Modified Fisher Grade (mFG)7
- mFG0: No SAH or IVH (intraventricular hemorrhage)
- mFG1: Minimal SAH and no IVH.
- mFG2: Minimal SAH with bilateral IVH.
- mFG3: Thick SAH (completely filling one or more cistern or fissure) without bilateral IVH.
- mFG4: Thick SAH with bilateral IVH.
- The cornerstone for the diagnosis of SAH is a noncontrast CT scan. The sensitivity of a head CT scan is highest in the first six hours after SAH and decreases to 58% by day 5.6
- A lumbar puncture (LP) should be performed if the CT scan is normal. The classic LP findings of SAH are elevated opening pressure, an elevated red blood cell count, and xanthochromia from hemoglobin breakdown products.
- Once the diagnosis of SAH is made, the etiology of hemorrhage must be determined with angiographic studies like digital subtraction angiography (DSA) or CT angiography.
Cardiac Complications (Electrocardiogram, Troponinemia, Neurogenic Stunned Myocardium)
- SAH can result in large reversible myocardial “stunning” injury.2,3
- Troponin levels commonly increase, typically less than the diagnostic threshold for myocardial infarction.
- Peak troponin levels correlate with the severity of neurologic injury and myocardial dysfunction.
- The mechanism is uncertain but is thought likely to be catecholamine-mediated.
- Severe, acute heart failure can develop similar to Takotsubo cardiomyopathy with ejection fractions commonly 10-30%. Often, there is akinesis at the apex and midventricle with the base preserved.
- Variant forms are also observed with akinesis in the midventricle or at the base5,8 (Figure 2).
- This phenomenon of neurogenic-stunned myocardium can cause severe limitation of cardiac contractility, which is reversible and associated with a good recovery profile.3
- Patients with SAH have echocardiogram changes similar to Takotsubo cardiomyopathy, which is also an acute transient dysfunction resulting from sympathetic overactivity.3
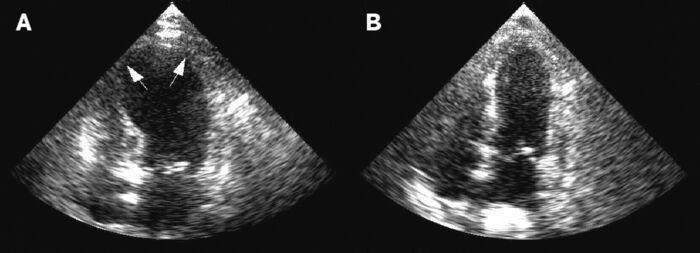
Figure 2. Stress-induced or Takotsubo cardiomyopathy is a complication commonly seen in aneurysmal subarachnoid hemorrhage. A: Apical hypokinesis and ballooning with reduced ejection fraction seen on echocardiography in the acute phase. B: Echo image showing resoution six days later. Source: Wikimedia commons. Gangadhar TC, et al. CC BY 2. Link.
- ECG shows the classic canyon T waves, nonspecific T-wave changes, QT prolongation, ST-segment depression, and U waves.3 (Figure 3)
- If ventricular function is adequate, ECG patterns other than those that are typical of myocardial ischemia are observed. They require no specific interventions or modifications of patient management other than attention to the possibility of dysrhythmias.
- Increased QT interval (greater than 550 milliseconds) frequently occurs after SAH, especially in patients with more severe SAH. Prolonged QT has been associated with an increased occurrence of malignant ventricular rhythms, including torsades de pointes.
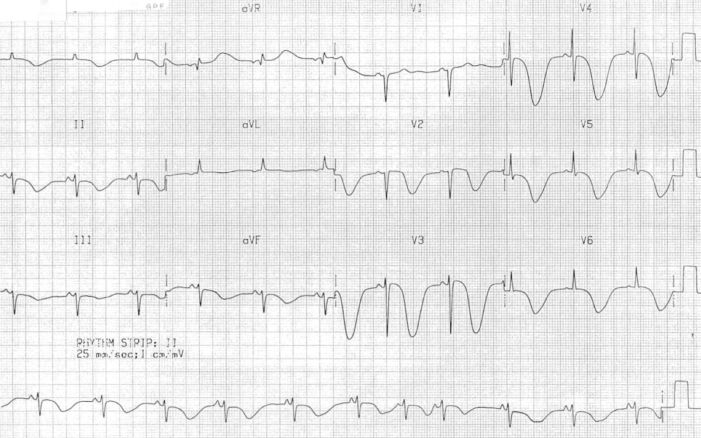
Figure 3. Giant canyon T-wave inversions in a patient with SAH. Source: Cadogan M, Buttner R. Life in the Fast Lane. CC BY NC-SA 4.0. Link.
References
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics- 2022 Update: A report from the American Heart Association. Circulation. 2022; 145(8): e153-639. PubMed
- Ko NU, Engelhard K. Neurocritical care. In: Gropper MA, Miller RD (eds). Miller's Anesthesia. 9th Edition. Elsevier, Philadelphia, PA, Elsevier. 2020. 2671-93.
- Lemkull BP, Drummond JC, Patel PM, et al. Anesthesia for neurologic surgery and neurointerventions. In: Gropper MA, Miller RD (eds). Miller's Anesthesia. 9th Edition. Elsevier, Philadelphia, PA, Elsevier. 2020. 1868–1910.
- Singer RJ, Ogilvy CS, Rordorf G. Aneurysmal subarachnoid hemorrhage: Epidemiology, risk factors and pathogenesis. In: Post T (ed). UpToDate. 2023. Link
- Sharma D. Perioperative management of aneurysmal subarachnoid hemorrhage: A narrative review. Anesthesiology. 2020; 133:1283–1305. PubMed
- Singer RJ, Ogilvy CS, Rordorf G. Aneurysmal subarachnoid hemorrhage: clinical manifestation and diagnosis. In: Post T (ed). UpToDate. 2023. Link
- Claassen J, Bernardini GL, Kreiter K, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: The Fisher scale revisited. Stroke 2001; 32:2012–20. PubMed
- Bihorac A, Ozrazgat-Baslanti T, Mahanna E, et al. Long-term outcomes for different forms of stress cardiomyopathy after surgical treatment for subarachnoid hemorrhage. Anesth Analg. 2016; 122 (5):1594-602. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.