Copy link
Anesthesia for Pituitary Surgery
Last updated: 03/06/2023
Key Points
- Pituitary tumors, or sellar masses, are either found incidentally on imaging or present with neurologic symptoms or hormonal abnormalities.
- Workup includes magnetic resonance imaging (MRI) with gadolinium and evaluation of hypothalamic-pituitary hormonal function with lab tests.
- Primary treatment is usually surgery or pharmacologic treatment. When these interventions fail or there is recurrence, radiation therapy is considered.
- Special anesthetic considerations are determined by the size, the surgical approach (e.g., transsphenoidal vs craniotomy), and whether hormonal abnormalities are present (e.g., need for exogenous hormone repletion, difficult airways and increased comorbidities).
The Hypothalamo-Pituitary-Adrenal Axis
- The hypothalamo-pituitary-adrenal axis is central to hormonal regulation.
- The specialized pituitary cells produce trophic hormones that in turn stimulate endocrine glands throughout the body. The produced hormones inhibit the release of their respective trophic hormones through a negative feedback loop (Figure 1).
- In certain pituitary adenomas the specialized cells proliferate and become autonomous resulting in overproduction of hormones leading to distinct endocrine syndromes.
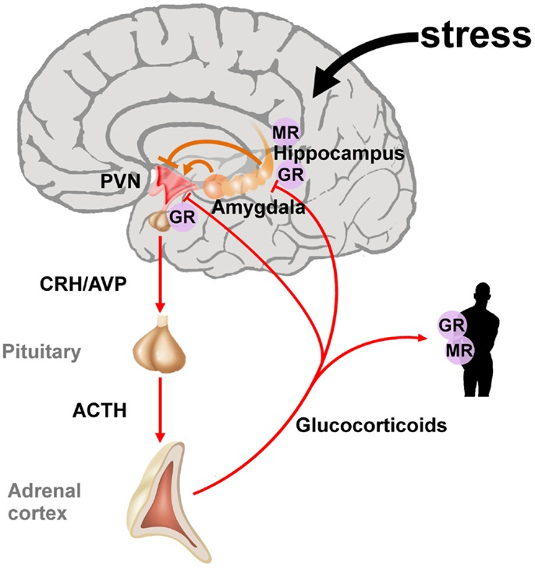
Figure 1. The hypothalamic-pituitary-adrenal axis integrates and mediates the stress response. Stressors can activate the paraventricular nucleus (PVN) of the hypothalamus directly or through projections from the amygdala. The hippocampus plays an important role in the assessment of stressors and as a site of glucocorticoid receptor (GR) mediated negative feedback regulation. Release of the hypothalamic neuropeptides corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) promotes the synthesis and secretion of adrenocorticotrophin (ACTH). ACTH in turn stimulates the release of glucocorticoids from the adrenal glands. These hormones circulate throughout the whole body and the brain and bind to intracellular nuclear steroid receptors. Hippocampal mineralocorticoid (MR) receptors act to the onset of the stress response, while GR at the hippocampus, PVN, and anterior pituitary terminates the stress response. Source: Wikimedia. Raabe FJ, Spengler D. CC BY 3.0. Link
Types of Pituitary Tumors
- Adenomas are most common benign tumors of the anterior pituitary and are classified by cell of origin and size at time of discovery. They commonly present with neurological symptoms, hormone imbalance, or are discovered incidentally.
- Pituitary adenomas can be classified based on their size and hormone secretion.1 Size at time of discovery:
- Macroadenoma (>1 cm)
- Microadenoma (<1 cm)
Hormone Secretion1
- Functioning adenoma: secretion of specific hormones causing symptoms of hormonal access (see “Clinical Presentation” section), usually presents as microadenoma.
- Nonfunctioning adenoma: silent growth, usually presents as macroadenoma with compressive symptoms (see “Clinical Presentation” section).
Other Tumor Types of the Sellar Region
- Craniopharyngioma: Histologically benign tumors that arise from remnants of Rathke’s pouch which present as growth retardation in children and visual changes in adults that can reach large size before symptoms related to mass effects.
- Meningiomas: Benign tumors that arise from the meninges which present as visual impairment and hormonal deficiencies.
- Malignancies: germ cell tumors, sarcomas, chordomas, carcinomas, lymphomas, metastases.
Clinical Presentation1,2
- Neurological symptoms due to mass effect (e.g., headache, nausea/vomiting, visual changes, hydrocephalus, growth retardation, Parinaud syndrome [decreased pupillary light reflex, paralysis of upgaze, retraction-convergence nystagmus])
- Incidental findings on imaging (clinically silent, mostly macroadenomas)
- Hormonal abnormalities (over or under production of hormones)
- Lactotroph adenomas (most common, up to 30% of all pituitary tumors) ➔ hyperprolactinemia ➔ hypogonadism in males and females
- Nonfunctioning or null-cell (second most common, up to 25%) ➔ can present with hormonal insufficiencies related to hypopituitarism.
- Corticotroph adenomas (10-15%) ➔ increased secretion of ACTH and cortisol ➔ classic Cushing’s disease (diagnosis: dexamethasone suppression test, and hypophyseal venous sampling).
- Somatotroph adenomas (5-10%)➔ increased growth hormone ➔ gigantism in children or acromegaly in adults.
- Gonadotroph adenomas (5) ➔ increased secretion of FSH and/or LH ➔ irregular menses, low testosterone, low libido, etc.
- Thyrotroph adenomas (extremely rare) ➔ increased secretion of TSH and T4 ➔ hyperthyroidism.
- Craniopharyngiomas ➔ hormonal deficiencies (e.g. diabetes insipidus, lack of growth hormone in children) .
- Most tumors can cause hyperprolactinemia after they have reached a certain size due to the so-called “stalk effect.” There is tonic inhibition of prolactin by dopamine that is transported through the stalk. Compression of the stalk removes this inhibition and prolactine production can increase even when there is no prolactinoma.
Workup
- Imaging: magnetic resonance imaging with gadolinium is the imaging modality of choice for sellar masses. Computed tomography can help with delineating bony structures of the skull base and facial skull.
- Laboratory workup: evaluate hypothalamic-pituitary hormonal function via labs such as serum prolactin, insulin-like growth factor, plasma ACTH, cortisol and 24-hr urinary free cortisol, total or free thyroxine and thyroid-stimulating hormone luteinizing hormone and follicular stimulating hormone and testosterone. Laboratory workup should include basic metabolic panel (to identify electrolyte and metabolic abnormalities) and complete blood count.1
Management
- Pharmacologic treatment to reduce hormonal secretion and hormonal effects of certain adenomas include1
- dopamine agonist (e.g., cabergoline, bromocriptine) for prolactinomas;
- somatostatin analog (e.g., octreotide) for gigantism and acromegaly; and
- propylthiouracil for hyperthyroidism.
- Transsphenoidal surgery is the primary treatment for most pituitary masses. Cerebrospinal fluid (CSF) leak may require local flap, abdominal or fascia lata free flap placement by surgeon.
- Radiation therapy should be considered when surgery and/or medications have been exhausted.
Anesthetic Considerations
Preoperative Evaluation and Management1,3
- Evaluation of pituitary function in patients with pituitary tumors before and after surgery Hormonal deficiencies may require preoperative replacement (e.g. hypothyroidism, adrenal insufficiency)
- Untreated hypothyroidism ➔ increased sensitivity to narcotics and barbiturates, prolonged emergence, myxedema coma
- Untreated adrenal insufficiency ➔ hemodynamic instability, hypotension, shock
- Patients with acromegaly and Cushing’s disease are at increased risk of hypertension, diabetes, cardiovascular disease and electrolyte abnormalities and additional preoperative workup (e.g., echocardiography, HbA1C) should be tailored accordingly.
- Careful airway evaluation is essential as airway management may be difficult in acromegaly and Cushing’s disease patients due to anatomic distortion and comorbidities such as obstructive sleep apnea and obesity.
Intraoperative Management1,2,3
- Monitoring: In addition to standard ASA monitors, arterial catheter should be considered for close blood pressure monitoring in anticipation of potentially sudden blood pressure changes. Precordial Doppler could also be considered because of a small risk of venous air embolism from patient positioning (slight head-up position to facilitate surgical access). Acromegalic patients may have ulner artery obstruction from ulnar artery compression at the wrist. Placement of radial artery catheter may therefore be inappropriate and alternative arterial sites should be considered.
- Patients are usually positioned slightly head-up and head is typically secured in a Mayfield headholder and the bed is usually turned 90 degrees with the right side away from the anesthesia team. Depending on the need for abdominal fat graft or fascia lata free flap, these areas will be prepped and draped (Figure 2).
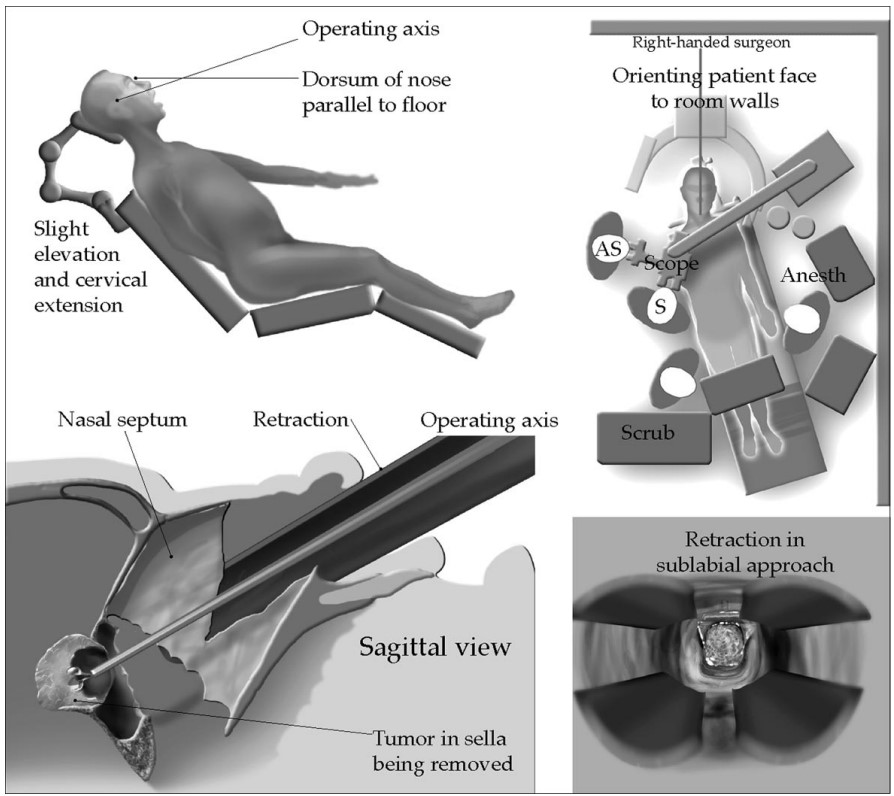
Figure 2. The patient is usually positioned slightly head up and the operating table is turned 90 degrees, which restricts the anesthesia team’s access to the patient. Typically a transnasal surgical approach is selected for most sellar tumors. Used with permission from Nemergut EC, et al. Perioperative management of patients underoing transsphenoidal pituitary surgery. Anesth Analg. 2005;101(4):1170-81.1
- Backup airway equipment such as video laryngoscopes and smaller endotracheal tubes (e.g. 6, 6.5) should be available.
- Unexpected hemodynamic changes should be anticipated (e.g. intranasal vasoconstrictor administration by surgical team, stimulation during endonasal approach, etc).
- Bleeding should be anticipated given the proximity of major blood vessels around the pituitary. Large bore peripheral intravenous (IV) access should always be considered. Central venous access is rarely necessary.
- Urine output and fluid balance monitoring is recommended especially for hormone secreting tumors (risk of electrolyte imbalance), or large tumors (risk of diabetes insipidus – see below).
- Balanced anesthetic technique using opioids and neuromuscular blockers is essential to negate any risk of movement and hemodynamic derangements.
- Total IV anesthetics may provide smooth emergence and reduce postoperative nausea and vomiting as compared with volatile anesthetics.
- Trans-sphenoidal approach requires special anesthetic considerations during emergence from anesthesia, e.g., avoidance of positive pressure ventilation, avoidance of cough on emergence, enhanced nausea and vomiting prophylaxis and strict nasal precautions.
- The use of remifentanil and dexmedetomidine infusions may be preferred to achieve such goals.
- Suctioning of stomach and hypopharynx should be performed by the surgical team prior to turning over airway to anesthesia team for airway management. Blind insertion of gastric tubes through the mouth is not recommended as this may accidentally pass through the skull base defect.
- Optimal ventilator management should be emphasized to preserve functional residual capacity to reduce hypoxia and minimize the need for supplemental oxygenation.
- When obstructive sleep apnea is a concern, nasopharyngeal airway placement and securement by the surgical team prior to handing over the airway to the anesthesia teams could be considered.
- When there is an increased risk of CSF leak, exchange of an endotracheal tube to a supraglottic airway (Bailey’s maneuver) maybe considered under deep anesthesia to reduce coughing on emergence.
Postoperative Management1,3
- Airway compromise may require immediate rescue with a supraglottic airway vs. intubation and avoidance of mask ventilation whenever possible. Pneumocephalus and infection may result from mask ventilation.
- Postoperatively, CSF leak precautions are needed (avoidance of coughing, straining and straws). CSF leaks when large may require reoperation and/or CSF diversion.
- Endocrinology consult for monitoring and managing of hormonal insufficiencies such as adrenal insufficiency, panhypopituiatirsm and diabetes insipidus should be considered.
- Monitoring for diabetes insipidus (DI) (injury to pituatiary stalk/posterior hypophysis):
- If urine output is greater than 200-250 mls/hour for two consecutive hours, perform diagnostic tests (paired urine/serum osmality, serum sodium, serum specific gravity) and monitor serum sodium every 4-6 hours. Diagnosis is based on low urine osmolality (<200 mOsm/L) while serum osmality is high (>310 mOsm/L) and low urine specific gravity (<1.005).1
- Treatment of DI is desmopressin acetate (DDAVP) (a synthetic analog of ADH) either 1 mg orally or 0.5 mcg subcutaneous as a single dose or every 12 hours until resolution of DI. Free water replacement may be required until DDAVP is available/administered.
- Stress dose steroid replacement is recommended for most cases, except for Cushing’s disease (ACTH secreting). In Cushing’s disease additional steroids may need to be withheld to avoid steroid excess and to ensure that cortisol levels have dropped. It may be appropriate to withhold additional steroids and evaluate the need for steroids through serial cortisol measurements. Endocrinology consult is recommended for guidance on perioperative steroid administration.
References
- Nemergut EC, Dumont AS, Barry UT, et al. Perioperative management of patients underoing transsphenoidal pituitary surgery. Anesth Analg. 2005;101(4):1170-81. PubMed
- Dunn LK, Nemergut EC. Anesthesia for transsphenoidal pituitary surgery. Curr Opin Anaesthesiol. 2013;26(5):549-54. PubMed
- Kim J, Scott-Miller R. Anesthesia for pituitary surgery. Otolaryngol Clin North Am. 2022;55(2):421-30. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.