Copy link
Sodium Nitroprusside
Last updated: 05/24/2023
Key Points
- Sodium nitroprusside (SNP) is a potent arterial and venous vasodilator used to treat acute hypertensive crises, acute decompensated heart failure, and to increase the cardiac output in the clinical setting of cardiogenic shock and high systemic vascular resistance (SVR), among other uses.1
- SNP has a rapid onset of action of 30 seconds, and the peak effect is seen within 2 minutes. The effects of SNP usually disappear 3 minutes after the drug is stopped.2
- Prolonged administration or high doses of SNP can result in toxicity from conversion of SNP to cyanide (CN) and thiocyanate. Methemoglobinemia can also occur.2
Introduction
- SNP is direct-acting, highly potent nonselective arterial and venous vasodilator given intravenously used to treat acute hypertensive crises, acute decompensated heart failure, and to increase cardiac output in the clinical setting of cardiogenic shock and high SVR, among other uses.1-3
- SNP has a rapid onset of action of 30 seconds, and the peak effect is seen within 2 minutes. Drug effects usually disappear 3 minutes after the drug is stopped.2
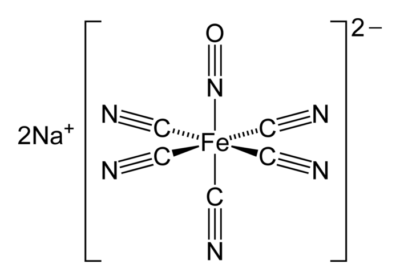
- SNP is a water-soluble sodium salt comprising a central ferrous ion complexed with a nitrosyl group and five cyanide (CN) ions2 (Figure 1).
Mechanism of Action
- Acting as a prodrug, SNP is a direct-acting, nonspecific vasodilator that interacts with sulfhydryl groups on erythrocytes to form methemoglobin and release cyanide and nitric oxide (NO).3
- NO binds to the vascular endothelium, activating guanylate cyclase and stimulating the cyclic GMP-protein kinase G (PKG) pathway. The cGMP inhibits calcium transport and produces the clinically therapeutic effect of vascular smooth muscle relaxation.2,3
- SNP causes comparatively balanced vasodilation within both the venous and arterial systems. It does not affect nonvascular smooth muscle such as the bowel and the uterus.2
- Of note, production of NO by vascular endothelial cells is often impaired in patients with hypertension. Unlike nitroglycerin, nitroprusside administration does not result in tolerance.1,2
Metabolism
- SNP interacts with oxyhemoglobin, transferring an electron from oxyhemoglobin to SNP.
- This rapid decomposition of the SNP radical causes release of therapeutic NO and five complexed CN ions.1,2
- Four of these CN ions are converted to thiocyanate in the liver through an enzymatic process involving rhodanese and thiosulfate as a sulfur donor.
- Thiocyanate is excreted in the urine, with a half-life elimination of roughly 3 days. This is prolonged in patients with renal dysfunction.3
- The remaining CN ion reacts with methemoglobin to form cyanmethemoglobin. When SNP infusions exceed the capacity of liver, or when sulfur stores or methemoglobin are depleted, CN radicals accumulate and can cause cyanide toxicity.2
Dosing
- SNP has a rapid onset of action within 30 seconds of IV dosing. Peak effect is usually seen within 2 minutes.3
- The duration of action is between 3-7 minutes with the effects of SNP ending 3 minutes after the infusion is stopped.3
- The elimination half-life of SNP is 1.5 minutes.3
- SNP must be protected from light. If exposed to light or alkaline conditions, it will decompose.
- Invasive monitoring is indicated when dosing SNP.2
- IV infusion can be started at doses of 0.5 mcg/kg/min and titrated to effect with a maximum dose of 10 mcg/kg/min. The maximum dose of 10 mcg/kg/min should be limited to a period of 10 minutes. If the blood pressure is not well controlled after 10 minutes, an alternative agent should be selected, to allow for titration of SNP to a lower, safer dose.2
- A dose of 0.25-1.5 mcg/kg/min is frequently sufficient to treat hypertensive patients.2
- The dose should not exceed 2 mcg/kg/min in pediatric patients.1
- Thiocyanate levels should be monitored in patients with renal dysfunction or those on IV infusions for more than 3 days.1
- When administering SNP, the drug should be protected from light due to the rapid conversion of SNP to aquapentacyanoferrate ion, which would readily cause release of hydrogen cyanide in the patient.4
Clinical Uses
- Discovered in 1850, SNP’s antihypertensive effects were first noted in 1929, and its clinical utility in the management of hypertension became established in the 1970s.3,4
- The widespread use of SNP has decreased, as new more selective drugs with a better safety profile have been developed.3
- Some clinical uses of SNP include:
- Hypertensive crises: These are defined as a systolic blood pressure ≥ 180 mmHg or diastolic blood pressure ≥ 110 mmHg. SNP is used to lower the blood pressure, including patients with intracerebral hemorrhage.1,2
- Cardiac surgery: Historically, SNP was used to control intraoperative blood pressure. Newer drugs associated with less hypotension are sometimes used instead of SNP, given the risk of toxicity when using SNP for prolonged periods of time.1,2
- Heart failure: SNP reduces afterload and improves left ventricular function in patients with acute heart decompensated heart failure.1,2
- Aortic surgery: SNP can be used during cross-clamping of the aorta to mitigate the hemodynamic effects of the cross-clamp.1,2
- Controlled hypotension: SNP can be used to reduce the blood pressure in surgical bleeding procedures.3
- Emerging applications include prevention and treatment of the “no-reflow” phenomenon during percutaneous coronary intervention and symptomatic treatment of schizophrenia.5
Side Effects
Cyanide Toxicity
- In 1991, the United States Food and Drug Administration updated the package insert for SNP, emphasizing the risk of cyanide toxicity.1,4
- Cyanide toxicity can occur from the conversion of SNP to cyanide and thiocyanate. The accumulation of cyanide leads to severe metabolic lactic acidosis (lactate > 10 mM) which can occur with higher-dosed infusions, such as 5 mcg/kg/min for prolonged periods of time. These patients may have mental status changes and seizures.3
- Treatment with sodium thiosulfate can minimize the likelihood of cyanide toxicity in patients receiving high doses of SNP.3
Thiocyanate Toxicity
- Thiocyanate toxicity is a rare complication. Symptoms are anorexia, nausea, fatigue, disorientation and toxic psychosis. Thiocyanate can be removed by dialysis.2,3
Methemoglobinemia
- Methemoglobinemia is unlikely to occur, even in patients with methemoglobin reductase deficiency. It should be considered in patients with impaired oxygenation and adequate cardiac output if being treated with SNP.3
System-Specific Side Effects
- Cardiovascular: severe hypotension, tachycardia, coronary steal, shortness of breath3
- Renal: There is potential decreased renal function secondary to hypotension. Renin release may occur secondary to hypotension leading to hypertension with SNP is discontinued.3
- CNS: SNP increases cerebral blood flow and cerebral blood volume. In the setting of decreased intracranial compliance, treatment with SNP could cause an increase in intracranial pressure. Headache and dizziness may also occur.3
- Pulmonary: decrease in partial pressure of oxygen (PaO2) secondary to attenuation of hypoxic pulmonary vasoconstriction3
- Endocrine: hypothyroidism.1
- Hematologic: SNP inhibits platelet aggregation at doses higher than 3 mcg/kg/min but it has not been shown to have an adverse clinical effect.3 Methemoglobinemia may occur.2
Special Considerations
- SNP is contraindicated in patients with inadequate cerebral perfusion, acute heart failure with low SVR, and vitamin B12 deficiency. SNP must not be used with phosphodiesterase inhibitors.
- SNP is also contraindicated in patients with Leber’s hereditary optic atrophy and tobacco amblyopia. These patients are deficient in rhodanese, an enzyme that detoxifies cyanide.
- SNP should be used with caution in the following settings:
- Patients with underlying hypovolemia or anemia, in the setting of myocardial infarction secondary to the possibility of coronary steal3
- Patients with hepatic impairment/failure as cyanide toxicity is a risk2
- Patients with renal dysfunction as thiocyanate toxicity may occur3
References
- Hottinger DG, Beebe DS, Kozhimannil T, et al. Sodium nitroprusside in 2014: A clinical concepts review. J Anaesthesiol Clin Pharmacol. 2014; 30(4):462-471. PubMed
- Eschenhagen T. Treatment of hypertension. In: Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 13th edition. New York, NY, McGraw-Hill Education LLC; 2018.
- Krall T, Ramsey J. Vasodilators. In: Pharmacology & Physiology in Anesthetic Practice. 6th edition. Philadelphia; Wolters Kluwer Health; 2021: 508-513.
- Friederich JA, Butterworth JF. Sodium nitroprusside: Twenty years and counting. Anesth Analg. 1995; 81 (1): 152-62. PubMed
- Holme MR, Sharman T. Sodium nitroprusside. In StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2023.
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.