Copy link
Myelomeningocele
Last updated: 01/19/2023
Key Points
- Myelomeningocele (MMC) is the most common neural tube defect associated with lifelong comorbidities, including Chiari II malformation, motor and sensory deficits in lower extremities, neurogenic bladder and bowel, and cognitive impairment.
- Almost all MMC patients develop a Chiari II malformation, often resulting in obstructive hydrocephalus requiring ventriculoperitoneal shunting.
- Anesthetic considerations for postnatal surgical repair include latex precautions, prone positioning, and avoidance of direct pressure on the lesion.
Introduction
- MMC is the most common form of neural tube defect (NTD) or spina bifida and is characterized by a cleft in the spinal column with a corresponding skin defect, resulting in exposed spinal cord and meninges (Figures 1 and 2).
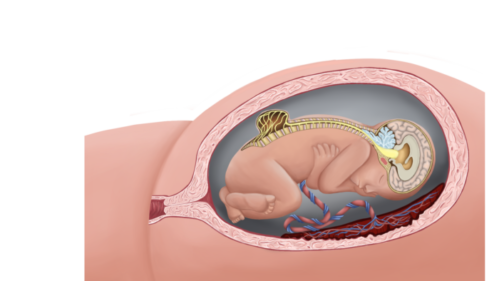
Figure 1. Lumbar myelomeningocele with Chiari II malformation (Used with permission from Colorado Fetal Care Center).
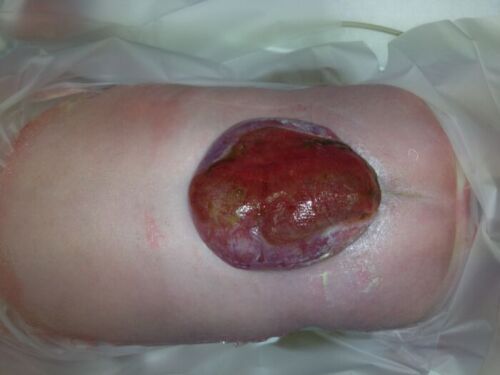
Figure 2. Lumbar myelomeningocele in a newborn positioned prone.
- MMCs are most commonly located in the lower lumbosacral region (90%).
- The incidence of NTDs is highly variable (<1 to 7 per 1000 live births) and depends upon geographic, ethnic, and nutritional factors. China, Ireland, United Kingdom, Pakistan, India, and Egypt have the highest incidence. In the United States, the prevalence is 0.3-0.4 per 1000 live births. Girls are more commonly affected than boys.
Etiology
Genetic Factors
- The recurrence risk of NTDs among siblings is 2-5% (20-50-fold increased risk).1 For a woman with a child with an NTD, the risk nearly triples after each affected pregnancy.
- Higher concordance rates are found in monozygotic twins and first-degree relatives.
Environmental Factors
- Folate deficiency is commonly associated with NTDs.
- Folic acid fortification of common foods (bread, flour, cereal, etc.) and folic acid supplementation have decreased the risk of NTDs. All women planning a pregnancy or capable of becoming pregnant should take 400 micrograms of folic acid daily.2
- Other risk factors include:
- Use of certain antiepileptic drugs (e.g., valproate, carbamazepine)
- Maternal fever in the first trimester of pregnancy
- Poorly controlled pregestational diabetes and prepregnancy obesity
- Lower socioeconomic status
- Exposure to environmental pollutants and pesticides
Pathophysiology
- MMCs are caused by a defect in primary neurulation, which occurs between the third and fourth weeks of gestation. Normally, the lateral edges of the neural plate become elevated to form the neural folds that subsequently fuse to form a closed neural tube.1
- According to a two-hit hypothesis, the primary hit is the defect in primary neurulation, and the second hit is the continued damage of the exposed spinal cord to amniotic fluid and mechanical trauma.
Prenatal Diagnosis - All pregnant women should be screened for NTDs between 18- and 22-weeks of gestation with either maternal serum alfa-fetoprotein (AFP) levels or ultrasonography.2
- AFP is a fetal-specific globulin that is synthesized by the fetal yolk sac, gastrointestinal tract, and liver and excreted into the amniotic fluid. Normally, the maternal serum AFP and amniotic fluid AFP levels peak between 12-14 weeks of gestation and then progressively decline.
- Maternal serum AFP levels are expressed as multiples of the median (MoM). Maternal serum AFP levels ≥ 2.0 or 2.5 MoM is considered abnormal.
- Widespread use of high-resolution ultrasonography in the second trimester allows the accurate diagnosis of myelomeningocele.2
- Spine: osseous defect in thoracic or lumbosacral spine, sac with neural elements (Figure 3)
- Cranial signs
- Ventriculomegaly, hindbrain herniation, and other features of Chiari II malformation
- Lemon sign: scalloping or concavity of the frontal bones (Figure 4)
- Banana sign: convex-shaped cerebellum from spinal cord tethering and downward migration of the cerebellum (Figure 5)
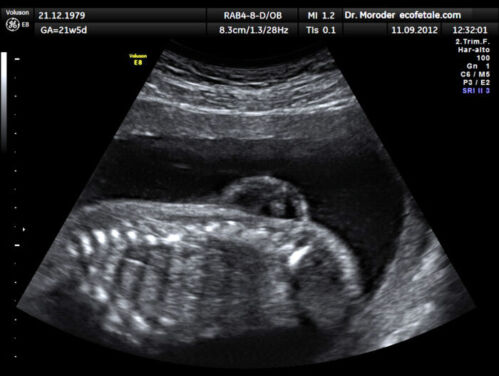
Figure 3. Lumbar myelomeningocele in a 21-week fetus (Source: Wolfgang Moroder, Wikimedia. CC BY SA 3.0)
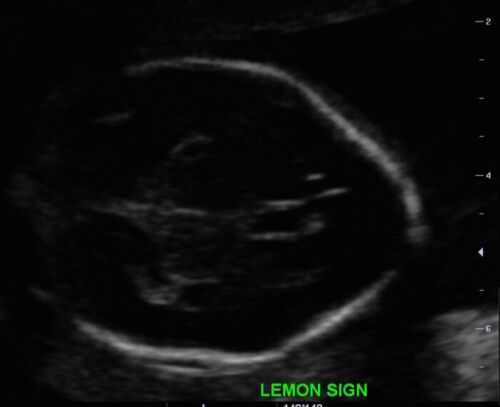
Figure 4. Lemon appearance of skill on antenatal ultrasound (Source: Case courtesy of Dr. Praveen Jha, Radiopaedia.org, rID:35151 CC BY SA 3.0)
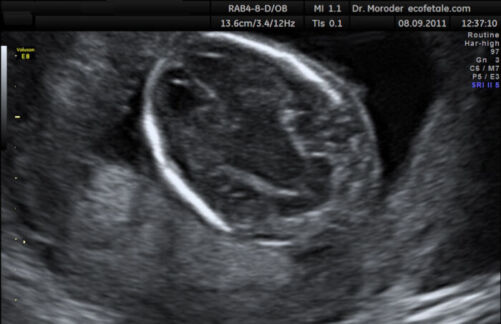
Figure 5. Banana shaped cerebellum on antenatal ultrasound (Source: Wolfgang Moroder, Wikimedia. CC BY SA 3.0)
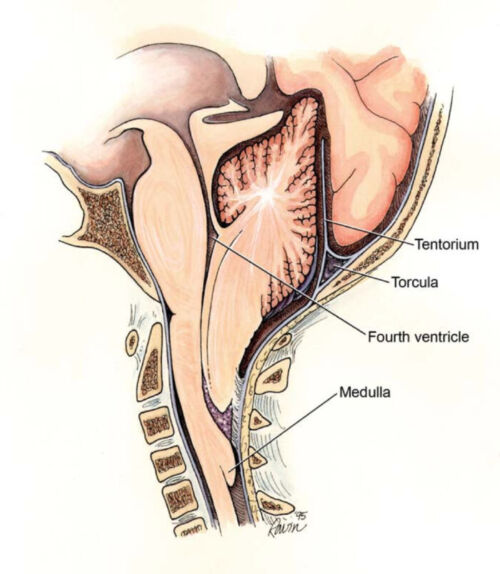
Figure 6. Chiari II malformation (Wikimedia Commons. CC BY 2.0)
Associated Anomalies
Chiari II malformation (also known as Arnold-Chiari malformation)3
- Almost all patients with MMC develop a Chiari II malformation, which is characterized by a small posterior fossa, downward herniation of the cerebellar tonsils through the foramen magnum, polymicrogyria, kinking of the brainstem, and tectal beaking (Figure 6).
- Chiari II malformation causes brainstem and cognitive dysfunction, swallowing difficulties, vocal cord paralysis, stridor, and apnea in these patients.
Obstructive Hydrocephalus
- Approximately 70-90% of patients with MMC develop obstructive hydrocephalus from the Chiari II malformation, requiring ventriculoperitoneal (VP) shunting and multiple revisions.
Neurological Deficits
- Depending on the level of the lesion, motor and sensory deficits often result in lower limb weakness or paralysis, wheelchair dependence, and lack of sensation that increases the risk of pressure sores.
Neurogenic Bladder and Bowel
- Almost all MMC patients have bladder dysfunction with incontinence and recurrent urinary tract infections, often requiring clean intermittent catheterization and possible vesicostomy.
- Most MMC patients develop fecal incontinence. Decreased bowel motility also causes constipation, and some patients require antegrade continence enema (ACE).
Other Issues
- Orthopedic: club foot, hip subluxation/dislocation, joint contractures, kyphoscoliosis, etc.
- Cognitive dysfunction: learning disability, attention deficit, social isolation
- Sexuality: precocious puberty in females, erectile dysfunction in males
Postnatal Care
- Following prenatal diagnosis, the patient should be referred to a center with a multidisciplinary team of specialists for counseling and delivery planning. There is no consensus on the preferred method of delivery.2
- After stabilization in the delivery room, the newborn must be positioned in the prone or lateral position to avoid pressure on the MMC lesion. A sterile, saline-soaked gauze should be used to cover the lesion, and prophylactic antibiotics should be started.5
- Latex precautions must be instituted from birth.
- Surgical closure should be performed within 72 hours after birth.5 Following surgical closure, the progression of hydrocephalus must be closely monitored, and some infants may need VP shunting.
Anesthetic Considerations for Postnatal Surgical Repair
Preoperative Investigations
- Complete blood cell count for baseline and type and crossmatch for surgery
- Basic metabolic profile to rule out electrolyte abnormalities or dehydration from large lesions
- Cranial ultrasound to assess the extent of ventriculomegaly and hydrocephalus
- Renal ultrasound to rule out genitourinary abnormalities
Intraoperative & Postoperative Care
- Continue latex precautions
- Most centers perform the surgical repair under general endotracheal anesthesia. However, the safe use of spinal anesthesia with hyperbaric 0.5% tetracaine with epinephrine injected into the caudal end of lumbar myelomeningoceles in a case series of 14 neonates has been described.6
- During induction, avoid direct pressure on the MMC lesion by using towels and donut rings to support the uninvolved portion of the infant’s back; alternatively, the infant may be positioned in the lateral decubitus position for induction and intubation.
- Standard ASA monitors, including temperature monitoring, must be used. Arterial and central lines are rarely used. Blood loss can be considerable for larger lesions, and appropriate intravenous access should be obtained.
- Surgical repair is performed in the prone position, and the infant must be appropriately positioned and padded.
- Postoperative ventilation in the prone position for 1-3 days is not uncommon.
References
- Copp AJ, Adzick NS, Chitty LS, et al. Spina bifida. Nat Rev Dis Primers. 2015.1:15007. PubMed
- Practice Bulletin No. 187. Neural tube defects. American College of Obstetricians and Gynecologists. Obstet Gyencol. 2017; 130: 2279-90. PubMed
- Coleman BG, Langer JE, Horii SC. The diagnostic features of spina bifida: The role of ultrasound. Fetal Diagn Ther. 2015;37: 179-96. PubMed
- McLone DG, Dias MS. The Chiari II malformation: cause and impact. Childs Nerv Syst. 2003.19:540-50. PubMed
- Burke R, Liptak GS. Providing a primary care medical home for children and youth with spina bifida. Pediatrics. 2011.128(6):1645-57. PubMed
- Viscomi CM, Abajian JC, Wald SL, et al. Spinal anesthesia for repair of myelomeningocele in neonates. Anesth Analg. 1995;81(3): 492-5. PubMed
Other References
- Chatterjee D. Myelomeningocele. OpenAnesthesia. Published January 2018. Accessed January 20, 2023. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.