Copy link
Chronic Pain: Methadone
Last updated: 03/14/2023
Key Points
- Methadone is a potent synthetic opioid that provides effective analgesic for postprocedural, cancer-related, and nociceptive pain.
- Other advantages include a tolerable side-effect profile, low cost, and potential for use in patients with impaired liver or kidney function.
- Additional high-quality evidence is needed to establish the role of methadone as an adjunctive medication or as monotherapy in chronic pain management.
Chronic Pain
- Chronic pain, defined as pain lasting greater than 3 months following initial tissue damage, is estimated to have a prevalence of approximately 20% among adults in the United States.1
- Chronic pain is often classified as chronic cancer-related pain and chronic noncancer pain, which is further subclassified as neuropathic or nociceptive. Since chronic pain often results from a combination of biological, social, and psychological factors, a multifactorial approach is required, which often includes the choice of an appropriate pharmacologic agents.
- Methadone is a strong opioid that is increasingly being considered for the management of both cancer-related and noncancer-related pain.2
Methadone: Pharmacology
- Methadone is a relatively inexpensive synthetic opioid that acts as a:
- potent μ-receptor agonist;
- N-methyl D-aspartate (NMDA) receptor antagonist; and
- an inhibitor of serotonin and norepinephrine reuptake (useful for neuropathic pain).
- After intravenous administration, methadone has a rapid onset of action (8 minutes), similar to fentanyl and sufentanil (5-6 minutes). After oral administration, the onset of analgesia is typically 30 minutes to 1 hour, peaking in 1 to 7.5 hours.
- It also has the longest half-life among the opioids used clinically. Methadone has a long elimination half-life of 24-36 hours, but a short redistribution half-life. After a single bolus dose, rapid redistribution terminates its analgesic effect, especially with small doses. When small doses of methadone are administered (5 -10 mg), it acts as shorter-acting opioid with a duration of action lasting for 3-4 hours. However, when larger doses are administered (20 mg), the clinical effect parallels the long elimination half-life (about 36 hours).
- The pharmacokinetics and side effects of methadone are listed in Table 1.
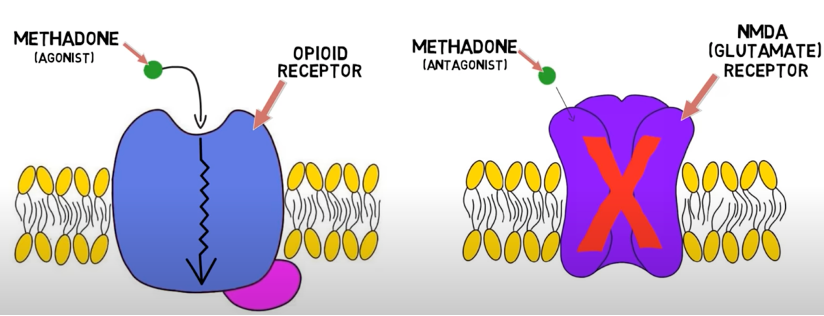
Figure 1. Mechanism of action of methadone. Source: Dingman M. 2-Minute Neuroscience: Methadone. Neuroscientifically Challenged. Link.

Table 1. Pharmacokinetics and side effects of methadone2
Methadone for Chronic Pain
- In chronic cancer pain, methadone is typically considered after treatment with one or more pure mu receptor agonists (i.e., morphine, oxycodone, hydromorphone, oxymorphone) has been ineffective.3
- In chronic noncancer pain, opioid therapy as a whole remains controversial due to the lack of evidence of long-term efficacy. If used, treatment should be initiated with immediate-release/short-acting agents in conjunction with nonpharmacologic therapies and nonopioid pharmacotherapy.4
- A systematic review in 2021 that included 40 randomized controlled trials demonstrated analgesic effectiveness of methadone in various types of pain, especially postprocedural, cancer-related and nociceptive pain.2
Perioperative Methadone Use
- Several clinical trials have documented the safe use of methadone for various surgical procedures such as posterior spinal fusion, major abdominal surgery, etc. Most studies reported a significant reduction in postoperative analgesic requirement, lower pain scores and no increase in opioid-related side effects.5
- The optimal dosing regimen for intraoperative methadone use has not been established. Most clinical trial used a dose of either 0.2 mg/kg or a fixed dose of 20 mg in adults after the induction of general anesthesia.5 Careful titration of additional methadone (3-5 mg at least 20 minutes between doses) can further prolong the duration of postoperative analgesia.
- Despite a favorable pharmacokinetic profile, some authors opine that there are several misperceptions regarding methadone and it is underutilized for perioperative analgesia.6
- Stigma plays a role as a barrier to treatment of chronic pain with methadone.7
Methadone for Opioid Use Disorders
- Secondary to its high bioavailability after oral administration and a long duration of effect, methadone is used along with buprenorphine for medication-assisted treatment of opioid use disorder (OUD).
- In the setting in which a patient endorses using methadone for OUD, certain “best practices” should be considered.8
- The methadone clinic should be contacted for dose and timing verification. Methadone for OUD is a single-dose administration, timed to the same time of day, each day. Typically, methadone clinic administration occurs between 5am-1pm daily.
- The name, number, dose, and time of administration should be documented in the electronic medical record for continuity of care purposes.
- Short-acting opioids should be utilized in addition to methadone daily administration for management of acute postsurgical pain. Partial opioid agonists should be avoided.
- Weaning patient off short-acting opioids prior to discharge should be considered as acute pain resolves to avoid relapse. Discharge with additional opioids may be considered a violation of their methadone clinic policies in some cases.
Methadone Black Box Warnings
- After an exponential increase in methadone deaths in noncancer pain patients in 2006, the Food and Drug Administrations issued a public safety advisory in 2006, which was followed by the following “black box warning” by the manufacturer:9
- “Deaths, cardiac and respiratory, have been reported during initiation and conversion of pain patients to methadone treatment from treatment with other opioid agonists. It is critical to understand the pharmacokinetics of methadone when converting patients from other opioids (see DOSAGE AND ADMINISTRATION). Particular vigilance is necessary during treatment initiation, during conversion from one opioid to another, and during dose titration. Respiratory depression is the chief hazard associated with methadone hydrochloride administration. Methadone’s peak respiratory depressant effects typically occur later, and persist longer than its peak analgesic effects, particularly in the early dosing period. These characteristics can contribute to cases of iatrogenic overdose, particularly during treatment initiation and dose titration.”
- In 2006, the FDA issued a safety alert regarding deaths and cardiac arrhythmias with methadone.9
- “In addition, cases of QT interval prolongation and serious arrhythmia (torsades de pointes) have been observed during treatment with methadone. Most cases involve patients being treated for pain with large, multiple daily doses of methadone, although cases have been reported in patients receiving doses commonly used for maintenance treatment of opioid addiction.”
- In 2009, an independent expert advisory panel to the FDA published the following guidelines for therapeutic QTc monitoring during methadone treatment.9
- Patients should be informed of arrhythmia risk.
- Clinicians should ask about any history of structural heart disease, arrhythmias, and syncope.
- A pretreatment electrocardiogram to measure the QTc interval and a follow-up ECG should be obtained within 30 days and annually.
- If the QTc interval is between 450 and 500 milliseconds, discuss the potential risks and benefits with the patient. If the QTc interval exceeds 500 milliseconds, discontinue, or reduce the methadone dose.
- Clinicians should watch out for interactions between methadone and other drugs that prolong the QT interval.
References
- Zelaya CE, Dahlhamer JM, Lucas JW, et al. Chronic pain and high-impact chronic pain among U.S. adults, 2019. NCHS Data Brief, no 390. Hyattsville, MD: National Center for Health Statistics. 2020. Link
- Hanna V, Senderovich H. Methadone in pain management: A systematic review. J Pain. 2021;22(3):233-45. PubMed
- Portenoy RK, Mehta Z, Ebtesam A. Cancer pain management with opioids: Optimizing Analgesia. In: Post T, ed. UpToDate; 2022. Accessed Nov 2022. Link
- Rosenquist R. Use of opioids in the management of chronic non-cancer pain. In: Post T, ed. UpToDate; 2022. Accessed Nov 2022. Link
- Murphy GS, Szokol JW. Intraoperative methadone in surgical patients. Anesthesiology. 2019; 131:678-92. PubMed
- Kharasch ED. Intraoperative methadone: rediscovery, reappraisal or reinvigoration? Anesth Analg. 2011;112(1):13-6. PubMed
- Shah S, Diwan S. Methadone: does stigma play a role as a barrier to treatment of chronic pain. Pain Physician. 2010;13(3):289-93. PubMed
- Ward EN, Quaye AM, Wilens TE. Opioid use disorders: perioperative management of a special population. Anesth Analg. 2018; 127(2): 539-47. PubMed
- Christie JM. Opioid prescribing: Methadone risk mitigation. APSF Newsletter. Spring 2011. Link
Other References
- Dingman M. 2 Minute Neuroscience: Methadone. Neuroscientifically Challenged. Published June 7, 2019. Accessed Jan 23rd, 2023. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.