Copy link
Ventricular Septal Defects
Last updated: 02/21/2023
Key Points
- Ventricular septal defect (VSD) is the most common form of congenital heart disease (CHD).
- The majority of VSDs close on their own with no intervention.
- If left untreated, a VSD can lead to pulmonary overcirculation, pulmonary hypertension, and shunt reversal (Eisenmenger syndrome).
Introduction
- VSDs are abnormal communications between the left and right ventricles of the heart and are the most common congenital anomaly, accounting for 50% of CHD.
- VSDs can occur as an isolated defect (20%), multiple defects, or part of a more complex CHD.
- VSDs are classified based on their location (Figure 1).
- Conoventricular (subarterial, subpulmonary, outlet, or infundibular) (5%) defects are located beneath the pulmonary valve within the outlet septum.
- Perimembranous (80%) defects are located in the membranous septum where the aortic, mitral, and tricuspid valves are in continuity.
- Inlet or canal (10%) defects are located inferior to the tricuspid valve.
- Muscular (5%) defects are located anywhere in the muscular septum and can be multiple with a “Swiss cheese” appearance.
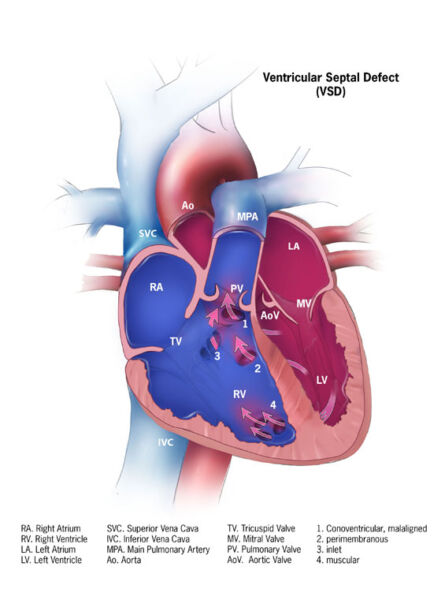
Figure 1. Types of VSDs: 1. conoventricular, 2. perimembranous, 3. inlet, 4. muscular. Source: Center for Disease Control and Prevention, National Center on Birth Defects, and Developmental disabilities.
Etiology
Embryology
- Failure of either complete formation or fusion of the five parts of the ventricular septum during embryologic development results in a VSD within the affected location.
Genetic Factors
- Chromosomal duplication or deletion disorders such as trisomy 21 (Down syndrome), 22q11 deletion (DiGeorge syndrome), and 45XO (Turner’s syndrome) are associated with VSDs.1
- Single gene disorders like the TBX5 mutation associated with Holt-Oram syndrome have also been found to play a role in VSD formation.
- Polygenic factors in which there are multiple genetic influences in VSD formation are thought to be common. Offspring of parents who have VSDs are more likely to be born with VSDs.
Environmental Factors
- Maternal exposure to teratogens, alcohol, drugs, and infections have been linked to VSDs.
- Maternal diabetes mellitus and untreated metabolic conditions are linked as well.
Pathophysiology
- The amount and direction of blood shunted through the VSD are dependent upon the size of the defect and the balance between pulmonary vascular resistance (PVR) and systemic vascular resistance (SVR).
- Shunt flow in large or “nonrestrictive” VSDs is dependent on the relative PVR:SVR and thus may vary over time. These shunts may be left-to-right, bidirectional, or right-to-left.
- Small or “restrictive” VSDs carry a high-pressure gradient but are not as easily manipulated by PVR: SVR.
- Isolated VSDs produce a left-to-right shunt during systole, leading to an increased volume load on the right ventricle, pulmonary artery, and ultimately the left atrium and ventricle.
- Over time, the increase in pulmonary blood flow (Qp) leads to an increase in PVR, and pulmonary hypertension (PH) can develop. As right-sided pressures increase, shunt reversal will occur, causing cyanosis (Eisenmenger’s syndrome).
Associated Anomalies
- VSDs are commonly part of a more complex set of cardiac defects, such as tetralogy of Fallot and transposition of the great arteries.
- Right and left-sided obstructive lesions are often present with VSDs. Right ventricular (RV) outflow obstruction is protective of the vascular bed with the risk of shunt reversal, whereas left ventricular (LV) outflow tract obstruction may worsen symptoms of increased Qp.
- Other common associations include VACTERL (vertebral, anal, cardiac, tracheoesophageal defects, renal, and limb abnormalities) and CHARGE (coloboma, heart defects, choanal atresia, retardation, genitourinary, ear abnormalities) syndromes.
Diagnosis
Presentation
- Patients presenting with a small restrictive VSD are usually asymptomatic. A pansystolic murmur may be heard on auscultation.
- Larger, nonrestrictive VSDs lead to congestive heart failure (CHF), tachypnea, tachycardia, pulmonary edema, feeding difficulties, and failure to thrive (FTT).2
- Infants born with a larger VSD may not become symptomatic until around 2-6 weeks of age when PVR has sufficiently fallen.
- Older children and adults who present late with larger VSDs may experience pulmonary hypertension (PH) and Eisenmenger’s syndrome.
Diagnostic Studies
- Transthoracic echocardiography (TTE) is the most commonly used modality to diagnose VSDs. TTE delineates the location, size, and number of defects, as well as other associated cardiac anomalies, and provides hemodynamic information such as estimated right-sided ventricular pressures (Figure 2).
- Cardiac magnetic resonance imaging (MRI) and computed tomography (CT) are generally reserved for VSDs associated with complex CHD and difficult anatomy that may not be well captured with TTE (Figure 3).
- Cardiac catheterization gives the most accurate pressure measurements and can assess the response to pulmonary vasodilators to guide therapy in patients with pulmonary vascular disease.
- Electrocardiography (ECG) is most likely normal in patients with restrictive VSDs. Larger shunts may show LV hypertrophy, right axis deviation, (RV) hypertrophy/strain and/or right bundle brand block.
- Chest radiography may show increased pulmonary vascular markings and an enlarged cardiac silhouette in patients with larger VSDs (Figure 4).
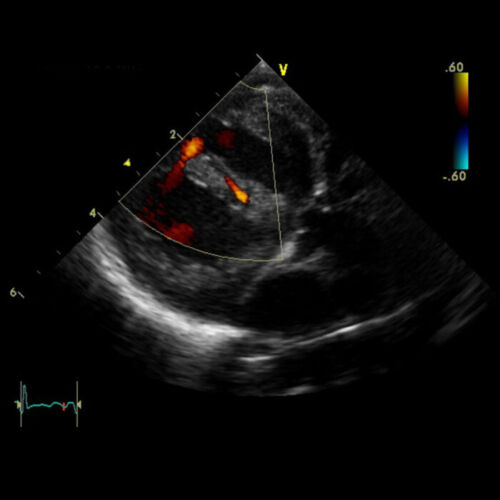
Figure 2. Transthoracic Echocardiogram demonstrating VSDs with left-to-right flow by color doppler in the parasternal long-axis view. Source: Case courtesy of Assoc Prof Frank Gaillard, Radiopaedia.org, rID: 7460. Link
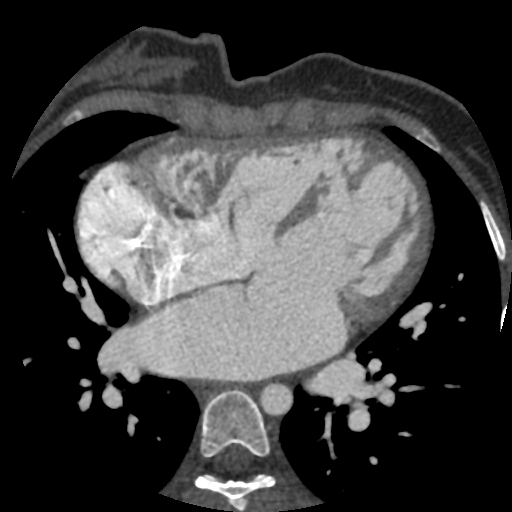
Figure 3. Cardiac MRI showing multiple VSDs in a patient with Goldenhaar syndrome. Source: Case courtesy of Dr Fabien Ho, Radiopaedia.org, rID: 73229. Link
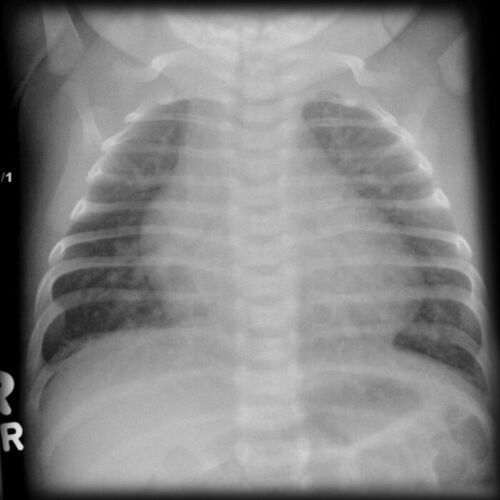
Figure 4. Chest radiograph showing cardiomegaly and increased pulmonary vascular markings. Source: Case courtesy of Assoc Prof Frank Gaillard, Radiopaedia.org, rID: 7445. Link
Treatment Strategies
General
- Most VSDs (80-90%) spontaneously close by about two years of age. VSDs that have not closed by age 2-4 years are electively closed to prevent disease progression.
- Infants younger than 6 months who are refractory to medical therapy and continue to exhibit signs and symptoms of CHF and FTT require VSD closure.
- Closure is also recommended in patients with Qp:Qs greater than 1.5 who are symptomatic, in patients with Qp:Qs greater than 2.0 and fluid overload, and in patients with VSD lesions larger than 5mm.
Medical Management
- Diuretics, digoxin, and angiotensin-converting enzyme inhibitors are used to manage patients with symptoms of CHF.
- Patients with pulmonary hypertension may require targeted therapy.
Percutaneous Device Closure
- Most muscular and some perimembranous VSDs are amendable to percutaneous device closure in the cardiac catheterization lab with high success and low complication rates.3
- Due to the proximity of subarterial or inlet VSDs to valvar tissue, these VSDs usually require surgical correction.
- Complications include failure, residual shunt, complete atrioventricular block (CAVB), cardiac arrhythmias, valvular regurgitation/disease, bleeding, embolic risk, and hematoma.
Surgical Management – Palliation and Repair
- A pulmonary artery band may be placed in symptomatic infants too small or ill to undergo surgical repair in order to limit Qp until there is sufficient growth.
- In VSDs not amendable to a transcatheter approach, surgical repair is performed via a median sternotomy using cardiopulmonary bypass.
- Perimembranous, inlet, and mid-muscular VSDs are accessed via a right atriotomy, whereas subarterial VSDs require a transpulmonary approach.
- Mortality is less than 1% following surgical closure, and complications include residual VSD, valvular incompetence, arrhythmias, LV dysfunction, complete heart block, and bleeding.
Anesthetic Considerations for Interventional/Surgical Management
Preoperative
Labs
- Complete blood cell count +/- coagulation studies
- Basal metabolic profile to assess renal function and electrolyte disturbances due to diuretic therapy
- Type and crossmatch
Imaging/Diagnostics
- ECG, echocardiography +/- MRI, CT
- Cardiac catheterization in patients with concern for pulmonary hypertension
- Chest radiograph
- +/- Head ultrasound to rule out intraventricular hemorrhage before heparinization in neonates
- +/- Doppler vascular structures to assess for access
Intraoperative
Monitoring
- Standard American Society of Anesthesiologists monitors
- Near-infrared spectroscopy
- Invasive pressure monitoring – central venous pressure, arterial pressure
- Transesophageal echocardiogram
Access
- Two large-bore peripheral intravenous (IV) catheters
- Arterial line
- Central line
Anesthesia Technique
- General endotracheal anesthesia is typically used.
- Air bubbles must be avoided in the IV lines.
- For surgical repair, regional (e.g., erector spinae nerve block) or neuraxial (e.g., caudal block) techniques may be performed for postoperative analgesia.
Hemodynamic Goals (induction, pre-CPB, post-CPB)
- Hemodynamic goals during induction and pre-bypass include maintaining a balance between PVR and SVR to minimize left-to-right shunting.
- Inhalational induction is usually well tolerated in patients with good ventricular function, normal RV pressures, mild symptoms, and no signs of PH. Patients may be hypovolemic due to diuretic therapy and may require additional fluids.
- Postbypass, there is generally not a great need for inotropic support, although low cardiac output syndrome may develop in patients with large unrestrictive VSDs and severe preoperative CHF. Dopamine, epinephrine, and/or milrinone may be used alone or in combination. Patients may require vasodilators such as nicardipine or nitroprusside for blood pressure control. Bleeding is not extensive, and blood products are usually not needed. If PH is present or develops acutely, inhaled nitric oxide may be used.
- Timing of extubation is dependent on preoperative symptoms but may be performed in the operating room in patients with smaller, less hemodynamically significant lesions.
Postoperative
- Bleeding, low cardiac output syndrome, arrhythmias (CAVB, right bundle branch block, junctional ectopic tachycardia), and pulmonary hypertensive crisis are potential complications.
- Subacute bacterial endocarditis prophylaxis is recommended for six months after surgical or percutaneous closure.
References
- Minette MS, Sahn DJ. Ventricular septal defect. Circulation. 2006; 114(20): 2190-7. PubMed
- Cox K, Algaze-Yojay C, Punn R, et al. The natural and unnatural history of ventricular septal defects presenting in infancy: an echocardiography-based review. J Am Soc Echocardiogr. 2020; 33(6): 763-70. PubMed
- Werynski P, Skored R, Wojcik A, et al. Recent achievements in transcatheter closure of ventricular septal defects: a systematic review of literature and a meta-analysis. Kardiol Pol. 2021; 79(2): 161-9. PubMed
Other References
- Ross FJ. Introduction to congenital heart disease. Part 1. OpenAnesthesia. Published April 1, 2018. Accessed February 21, 2023. Introduction to Congenital Heart Disease, Part 1
- Huffmyer J. Ventricular septal defects in adults. OpenAnesthesia. Published February 1, 2016. Accessed February 21, 2023. Ventricular Septal Defect in Adults
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.