Copy link
Aortic Stenosis: Hemodynamic Management, Comorbidities, and Treatment
Last updated: 06/13/2023
Key Points
- The goals of intraoperative management for patients with aortic stenosis (AS) include maintaining systemic vascular resistance, coronary perfusion pressure, and cardiac output.
- Commonly associated comorbidities include hypertension, chronic kidney disease, hypercholesterolemia, left ventricular systolic dysfunction, and atrial fibrillation.
- Treatment options include surveillance monitoring, medical management, balloon aortic valvuloplasty, surgical aortic valve replacement, and transcatheter aortic valve implantation.
Hemodynamic Management
- Depending on the severity of AS, perioperative hemodynamic monitoring should include standard American Society of Anesthesiologists (ASA) monitors and an arterial catheter for continuous blood pressure monitoring, central venous access for administration of vasoconstrictor therapy, and, if possible, intraoperative transesophageal echocardiography to assess left ventricular filling and contractility. Pulmonary artery catheters are not indicated in the absence of concomitant severe pulmonary hypertension.1
- Perioperative hemodynamic goals for AS include maintaining preload, afterload, and contractility, normal heart rate and sinus rhythm.1,2
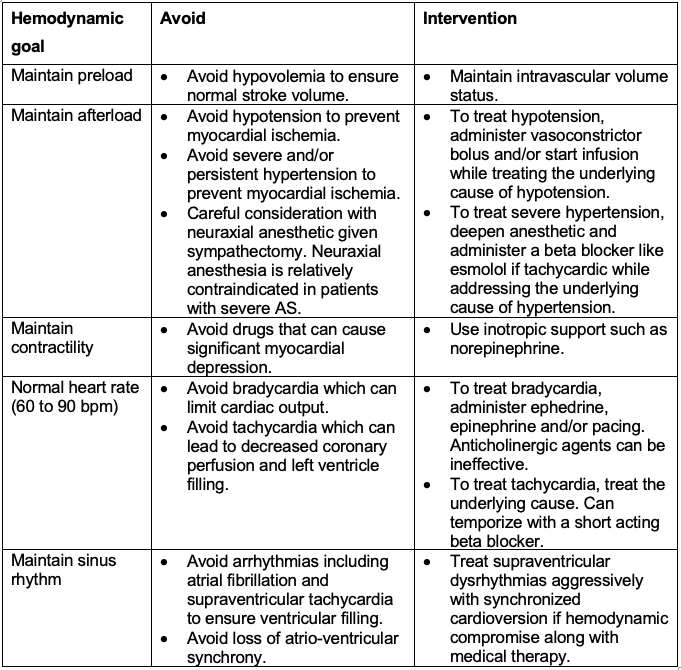
Table 1. Perioperative hemodynamic goals for aortic stenosis. Adapted from Townsley MM, Martin DE. A Practical Approach to Cardiac Anesthesia. 20131 and Fontes ML. UpToDate. 2022.2
Associated Comorbidities
- Associated comorbidities for AS can be divided into noncardiac and cardiac comorbidities.
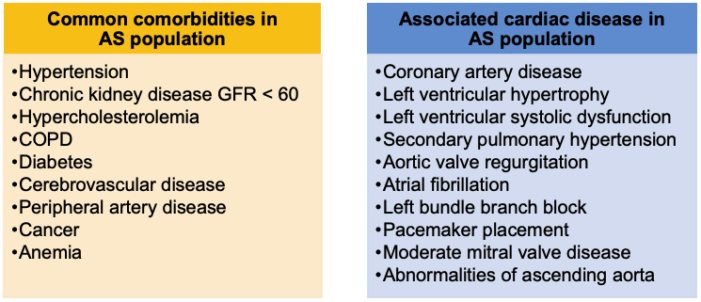
Table 2. Associated comorbidities in AS population. Adapted from Faggiano P, Frattini S, et al. International Journal of Cardiology. 2012.3
Medical Management
- Patients with asymptomatic AS should have their disease monitored with echocardiography, cardiovascular risk factors and comorbidities treated, and be educated on the early detection of symptom onset.
- Once symptoms appear (including dyspnea, angina, or syncope), surgical management should be considered.4
- Medical management for patients with symptomatic AS who have not undergone valve replacement, include treatment of concurrent cardiovascular conditions, prevention or treatment of superimposed diseases, maintenance of optimal hemodynamic conditions, and treatment of symptoms.4
- Balloon aortic valvuloplasty may be considered as a temporizing option in addition to optimal medical management in nonsurgical candidates (Table 3).1,4
Surgical Management
- Surgical interventions include transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement (SAVR).4,5
- Timing of surgical intervention for severe AS differs between asymptomatic and symptomatic patients.4,5
- Indications for valve replacement in asymptomatic patients with severe AS include
- left ventricular ejection fraction less than 50%;
- very severe AS, or;
- fall in systemic blood pressure with exercise.
- Indications for valve replacement in symptomatic patients with severe AS include
- life expectancy with AVR more than 1 year; and
- patient’s quality of life is likely to improve with SAVR or TAVI.
- Indications for valve replacement in asymptomatic patients with severe AS include
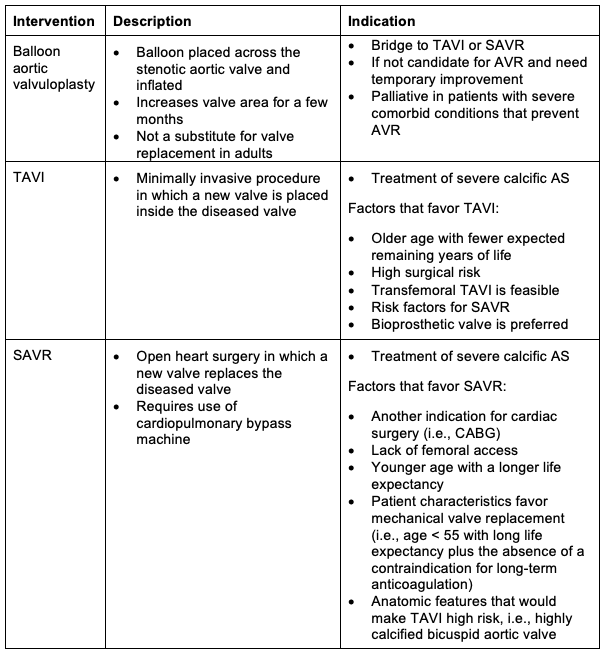
Table 3. Surgical Management for AS population. Adapted from Otto CM, et al. Circulation. 20214 and Vandvik PO, et al. BMJ. 2016.5
References
- Townsley MM, Martin DE. Anesthetic management for the surgical treatment of valvular heart disease. In: Hensley F, Martin DE, Gravlee GP. A Practical Approach to Cardiac Anesthesia. Fifth edition. Philadelphia, PA; Lippincott Williams and Wilkins; 2013:498-505.
- Fontes ML. Intraoperative hemodynamic management of aortic or mitral valve disease in adults. UpToDate; 2022. Accessed Oct 18, 2022. Link
- Faggiano P, Frattini S, Zilioli V, et al. Prevalence of comorbidities and associated cardiac diseases in patients with valve aortic stenosis. Potential implications for the decision-making process. Int J Cardiol. 2012;159(2):94-9. PubMed
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143: e90-103. PubMed
- Vandvik PO, Otto CM, Siemieniuk RA, et al. Transcatheter or surgical aortic valve replacement for patients with severe, symptomatic, aortic stenosis at low to intermediate surgical risk: a clinical practice guideline. BMJ. 2016;354. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.