Copy link
Glucose Homeostasis and Insulin Therapy
Last updated: 08/15/2023
Key Points
- Patients with type 1 diabetes mellitus (T1D) require insulin at all times, even while fasting, to maintain euglycemia and prevent ketosis.
- Insulin is most commonly administered subcutaneously (SC) but may be administered intravenously (IV) in hospitalized, critical patients.
- Devices for continuous insulin delivery and glucose monitoring are commonly used by patients with T1D.
Glucose Homeostasis
- Glucose metabolism is controlled by the opposing effects of insulin and counter-regulatory hormones (glucagon, catecholamines, cortisol, and growth hormone). Insulin increases glucose transport into cells, increases the synthesis of energy storage molecules, and suppresses endogenous glucose production. In contrast, glucagon increases endogenous glucose production and stimulates alternative metabolic pathways (e.g., lipolysis) when glucose is deficient.1
- Excess insulin results in hypoglycemia. Hypoglycemia presents with signs of autonomic activation (shakiness, sweating, pallor, palpitations) and neurologic dysfunction (confusion, altered vision, hearing, or speech, and lethargy). The blood glucose (BG) level at which patients feel symptoms of hypoglycemia varies by patient.2
- Insulin deficiency leads to hyperglycemia with volume depletion and electrolyte abnormalities (Figure 1). In the complete absence of insulin, glucagon activity is unchecked, leading to ketone body formation and metabolic acidosis. Diagnostic criteria for diabetic ketoacidosis (DKA) include hyperglycemia (BG greater than 200 mg/dL), acidosis (venous pH less than 7.3 or serum bicarbonate less than 18 mmol/L), and evidence of ketosis (serum or urine assessment). DKA is a medical emergency that carries a risk of mortality and significant morbidity, most commonly due to cerebral injury.3
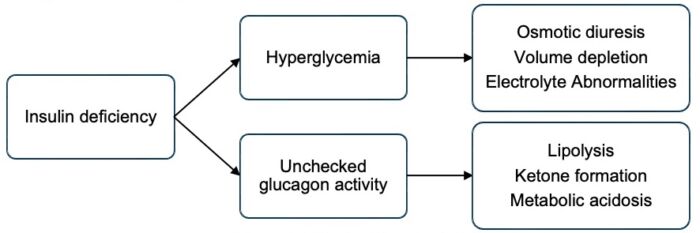
Figure 1. Effects of insulin deficiency
Insulin Therapy
- In the setting of insulin deficiency, exogenous insulin is used to maintain euglycemia and metabolic stability. Patients with absolute insulin deficiency (e.g., T1D) require continuous insulin administration, even while fasting, to meet metabolic demands. Those with relative insulin deficiency (e.g., T2D or physiologic stressors such as infection, surgery, or trauma) become hyperglycemic but typically have enough insulin activity to prevent ketosis.3
SC Insulin Therapy
- The goal of insulin therapy is to mimic physiologic insulin secretion, which occurs in two phases. Basal secretion is a continuous, low-level secretion that maintains metabolic stability. Prandial secretion is an intermittent, high-level secretion that maintains BG within a tight range after meals.4 Patients commonly achieve this pattern with one of two SC insulin dosing regimens, which differ in how the basal insulin is administered (Table 1).

Table 1. Subcutaneous insulin dosing regimens
IV Insulin Therapy
- IV insulin therapy can be used for glucose management in hospitalized patients. Only regular insulin is administered IV. The half-life of IV regular insulin is approximately 5 minutes, which allows for rapid titration in critically ill patients.5
Common Insulin Formulations5
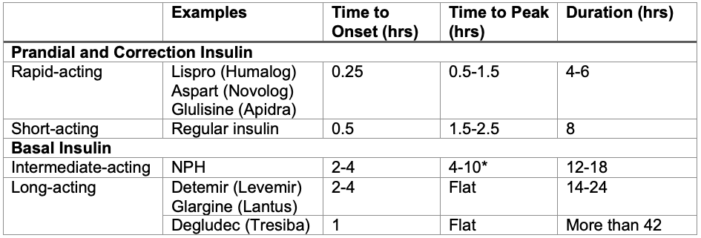
Table 2. Common insulin formulations. *NPH (neutral protamine Hagedorn) insulin is the only basal insulin with a broad peak of action. This may place patients at risk for hypoglycemia while fasting.6
Insulin Dosing
- Daily insulin dose requirements vary by patient. In general, prepubertal children require 0.7-1 IU/kg/day. Insulin requirements increase during puberty (1-2 IU/kg/day) and then decline. Approximately 30-50% of the daily insulin dose is given as basal insulin, with the remainder given as prandial or correction insulin doses. A patient’s insulin requirements can be affected by physiologic stressors (such as illness or trauma), fasting, and activity level.4
SC Insulin Bolus Dosing4,5
- SC insulin bolus requirements are calculated using the following formulas. These numbers are patient-specific, though exact insulin requirements may be altered in the perioperative period. SC insulin boluses should not be administered more often than every 3 hours due to the risk of dose-stacking with subsequent hypoglycemia.
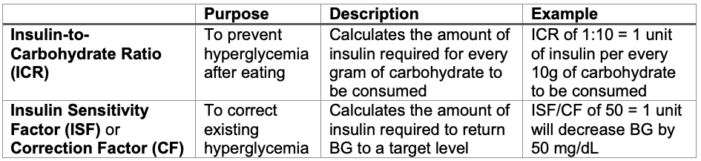
Table 3. Insulin bolus calculations
IV Insulin Dosing
Hospitalized patients may require IV insulin therapy, especially if critically ill or undergoing surgery. Several factors must be addressed to safely convert between SC and IV insulin administration.
- Patients receiving IV insulin should also receive dextrose-containing IV fluids to prevent hypoglycemia. These should be coadministered through the same infusion line.
- IV insulin has a much more rapid onset and offset compared to SC insulin.
- Converting from IV to SC insulin: IV insulin requirements may initially be decreased if the patient’s previously administered SC insulin is still active.
- Converting from SC to IV insulin: SC insulin should be administered at least 15 minutes before stopping the IV insulin infusion to prevent hyperglycemia.
- For patients using a continuous SC insulin infusion (CSII), IV insulin can be started at the patient’s basal rate. However, caution is required when converting from SC to IV insulin dosing because the dosing units are not the same. SC insulin is NOT weight-based (units/hr), while IV insulin is weight-based (units/kg/hr).
- If the patient’s basal insulin rate is unknown, the chart in Table 4 can be used to guide initial dosing.6 However, the chart is based on dosing regimens for patients who are critically ill or experiencing diabetic ketoacidosis. It may overestimate the insulin needs of surgical patients who are otherwise well-controlled and metabolically stable.
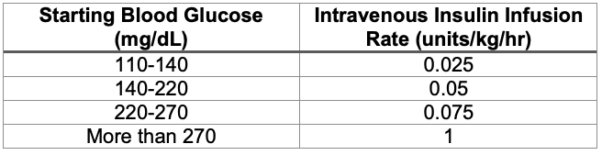
Table 4. Suggested intravenous insulin dosing regimen
- The IV insulin infusion rate should be titrated by 0.01-0.03 units/kg/hr to maintain euglycemia. IV insulin boluses should NOT be administered.
Diabetes Devices
- Medical devices, including insulin pumps and continuous glucose monitors (CGMs), are used to manage blood glucose in patients with T1D. The most recent International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines recommend the use of insulin pump therapy and continuous glucose monitoring for all children with T1D.7,8
Insulin Pumps
- Insulin pumps are mechanical devices that deliver a continuous SC insulin infusion (CSII) to meet basal demands and additional boluses of insulin to prevent or correct hyperglycemia. The pumps deliver only short-acting insulin. Because no long-acting insulin is used, the patient will have no basal coverage if the insulin pump is stopped. BG will start to rise within 30 minutes of stopping an insulin pump9 and patients can develop ketosis within 2 hours10 if an alternate basal insulin is not provided.
Insulin Pump Systems
- Tubed Insulin Pumps: The traditional insulin pump design consists of a pump and insulin reservoir contained in a single device. The device is connected by small-bore plastic tubing to a plastic cannula or metal needle that is placed SC at the insertion site. The pump is worn on the patient. The pump itself is designed to last for years, while the insulin reservoir, tubing, and insertion site are changed every few days. The pump can be temporarily disconnected from the patient while leaving the plastic or metal insertion site intact.
- Tubeless Insulin Pumps: Tubeless pumps consist of a disposable pump and reservoir that are placed directly at the insertion site. The pump actions are controlled remotely by a separate handheld device or smartphone app. Patients using these systems will replace the pump every few days, though the controller device is long-lasting. Tubeless pumps are similar in appearance to some CGMs and can be easily misidentified by personnel who are not familiar with them.
CGMs
- CGMs are devices that provide near-continuous glucose readings. CGMs consist of a SC filament and transmitter that are placed directly on a patient’s skin. The filament measures interstitial glucose concentrations every 1-15 minutes. The data is either transmitted automatically to a display device or smartphone (real-time CGM) or displayed when the patient scans the CGM with a reader (intermittently scanned CGM).8 The CGM filament and adhesive are changed every 7-14 days. Depending on the device, the transmitter portion is either disposable or used for several months.
- CGMs measure glucose values of SC fluid, which may lag behind serum values by 5-10 minutes. This may limit CGM accuracy when the patient is hypoglycemic or when BG values are changing rapidly.8 In addition, sensor accuracy can be affected by pressure on the sensor due to patient positioning (compression artifact), alterations in temperature or blood pressure, or exposure to certain medications. Acetaminophen exposure, in particular, was noted to artificially elevate CGM readings from older models, though this is less common with new models. The effects of commonly used anesthetic medication on sensor accuracy are unknown.5,6
Closed Loop Insulin Delivery Systems
- Closed loop insulin delivery, also called automated insulin delivery or “artificial pancreas” systems, refers to software that allows insulin pumps and CGMs to communicate wirelessly. Automated changes to insulin administration are made in response to BG values based on predictive algorithms. These systems will automatically adjust or pause the patient’s basal rate and administer boluses to maintain euglycemia without user input.7
Complications from Insulin Therapy
Hypoglycemia
- Excess insulin administration results in hypoglycemia. Hypoglycemia should be treated when BG is less than 70 mg/dL or if the patient is symptomatic (Figure 2).2 The priority for treatment should be administering additional glucose, rather than holding insulin, because of the risk of ketosis if basal insulin is held for a prolonged time. If glucose cannot be administered, BG is less than 60 mg/dL, or the patient has neurologic impairment, glucagon should be administered and/or insulin should be held.
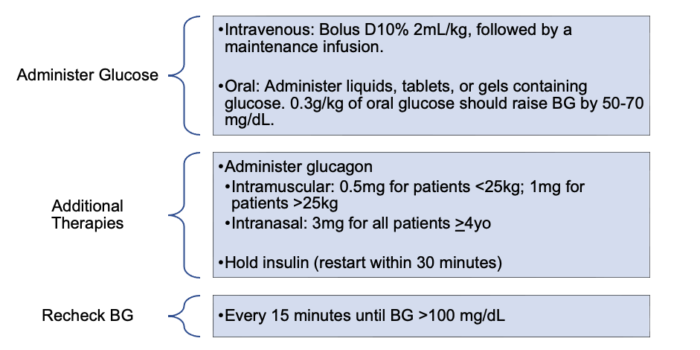
Figure 2. Management of hypoglycemia2,5
Hyperglycemia/DKA
- Insufficient insulin administration results in hyperglycemia and, potentially, DKA. Insulin should be administered to return BG to the patient’s target level (Figure 2). In the perioperative period, BG should be maintained within a target range of 90-180mg/dL. In addition, any BG greater than 250 mg/dL should trigger an evaluation for DKA.5 Treatment protocols for DKA are complex and outside the scope of this summary.
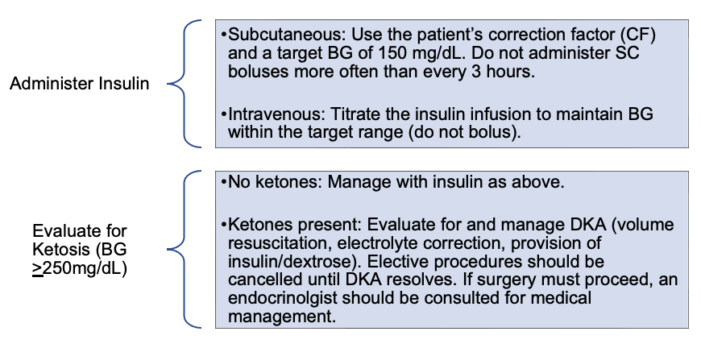
Figure 3. Management of hyperglycemia3,5
Insulin Pump Malfunction5
- Any unexplained metabolic instability (dysglycemia or ketosis) or pump alarms should raise concern for pump malfunction. In the event of pump malfunction, the pump should be turned off and physically disconnected from the patient. An IV insulin infusion should be started.
References
- Niswender KD. Basal insulin: Physiology, pharmacology, and clinical implications. Postgrad Med. 2011;123(4):17-26. PubMed
- Abraham MB, Karges B, Dovc K, et al. ISPAD clinical practice consensus guidelines 2022: Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2022;23(8):1322-40. PubMed
- Glaser N, Fritsch M, Priyambada L, et al. ISPAD clinical practice consensus guidelines 2022: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2022;23(7):835-56. PubMed
- Cengiz E, Danne T, Ahmad T, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Insulin treatment in children and adolescents with diabetes. Pediatr Diabetes. 2022;23(8):1277-96. PubMed
- Martin LD, Hoagland MA, Rhodes ET, et al. Perioperative management of pediatric patients with type 1 diabetes mellitus: Updated recommendations for anesthesiologists. Anesth Analg. 2020;130(4):821-7. PubMed
- Kapellen T, Agwu J, Martin L, et al. ISPAD clinical practice consensus guidelines 2022: Management of children and adolescents with diabetes requiring surgery. Pediatr Diabetes. 2022;23(8):1468-77. PubMed
- Sherr JL, Schoeler M, Dos Santos TJ, et al. ISPAD clinical practice consensus guidelines 2022: Diabetes technologies: Insulin delivery. Pediatr Diabetes. 2022;23(8):1406-31. PubMed
- Tauschmann M, Forlenza G, Hood K, et al. ISPAD clinical practice consensus guidelines 2022: Diabetes technologies: Glucose monitoring. Pediatr Diabetes. 2022;23(8):1390–1405. PubMed
- Zisser H. Quantifying the impact of a short-interval interruption of insulin-pump infusion sets on glycemic excursions. Diabetes Care. 2008;31(2):238-9. PubMed
- Orsini-Federici M, Akwi A, Canonico V, et al. Early detection of insulin deprivation in continuous subcutaneous insulin infusion-treated patients with type 1 diabetes. Diabetes Technol Ther. 2006;8(1):67-75. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.