Copy link
Perioperative Anemia
Last updated: 01/27/2023
Key Points
- Anemia is a prevalent problem in adult surgical patients and is a modifiable risk factor for poor perioperative outcomes. The administration of allogeneic blood is associated with inferior outcomes in surgical patients with anemia. Thus, transfusion is not an optimal treatment for preoperative anemia.1
- A comprehensive approach should be employed to prevent, diagnose, and treat anemia as part of patient blood management throughout a patient’s perioperative course.
Introduction
- Perioperative anemia is common, affecting about 25-75% of adult surgical patients.1 Anemia has been identified as an independent and modifiable risk factor for poor perioperative outcomes.1,2
- In surgical patients with anemia, the administration of allogeneic blood is associated with inferior outcomes.1 Thus, transfusion is not an optimal treatment for preoperative anemia.1
- Instead, a targeted approach should be employed to prevent, diagnose, and treat anemia as a part of the comprehensive patient blood management during the perioperative course.
- A commonly accepted definition of anemia in adults is a hemoglobin concentration less than 13 g/dL for all genders, as this value is associated with an increased risk of erythrocyte transfusion and associated adverse outcomes.2
Complications
- The presence of chronic preoperative and acute intraoperative anemia is a strong predictor of postoperative mortality after both cardiac and noncardiac surgery.2
- Anemia decreases arterial oxygen content (CaO2) and limits oxygen delivery to the organs and tissues, culminating in end-organ dysfunction/failure if left untreated.
- Notable complications of perioperative anemia include2
- acute kidney injury;
- stroke;
- myocardial injury and infarction.
- A retrospective study of over 227,000 surgical patients from the American College of Surgeons National Surgical Quality Improvement Program database showed that patients with anemia had increased adjusted odds ratio for mortality (1.42x), cardiac injury (1.45x), respiratory dysfunction (1.33x), and renal injury (1.37x). Another study from the International Surgical Outcomes Study database of approximately 38,000 patients showed that patients with moderate anemia (8.0-9.0 g/dL) had an increased risk of in-hospital mortality and myocardial infarction.2
- Further studies have demonstrated that the degree of anemia is proportional to the risk of poor perioperative outcomes.2
Preoperative Testing and Management
The presence of preoperative anemia, coagulopathy, and surgical factors that increase the risk of perioperative bleeding should be addressed during all preanesthetic evaluations.
Tenets of Preoperative Testing
- Treatments for anemia require time for maximum efficacy. Thus, testing should be completed at least 4 weeks prior to surgical intervention.1
- Screening for preoperative anemia with a complete blood cell count should be limited to:3
- patients undergoing major surgical procedures associated with large volume blood loss (> 10% chance of needing a transfusion or > 500 mL estimated blood loss);
- patients that are expected to have preoperative anemia unless the surgery to be performed is minor in nature.
- A step-by-step approach to determine the etiology of anemia and the guiding principles for the treatment of perioperative anemia are listed in Figure 1.
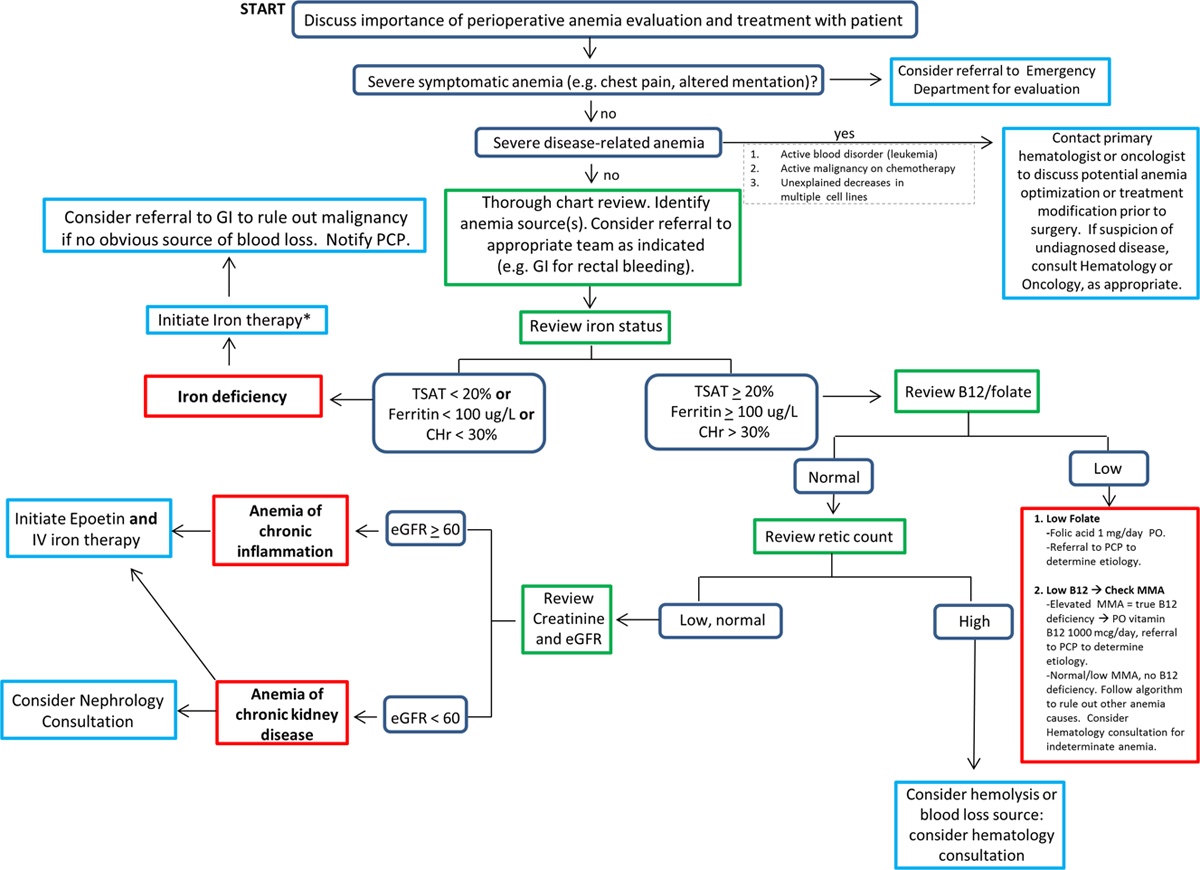
Figure 1. Algorithm for determining the etiology and guiding principles for the treatment of perioperative anemia. Reproduced with permission from Warner MA et al. Anesth Analg. 2020.1 Abbreviations: CHr = reticulocyte hemoglobin content, eGFR = estimated glomerular filtration rate, GI = gastrointestinal, IV = intravenous, MMA = methylmalonic acid, PCP = primary care provider, PO = per os, TSAT = transferrin saturation.
Iron Deficiency Anemia
- Iron deficiency anemia (IDA) is the most common cause of perioperative anemia and iron studies should be performed first.
Treatment of Iron Deficiency Anemia
- Iron replacement
- If laboratory studies show reductions in iron, ferritin, or CHr, then iron supplementation is indicated. The source of iron loss should be determined.
- The choice of oral vs. intravenous iron administration is based on several factors including;
- patient preference, timing of surgery, severity of anemia, ability to tolerate the side effects of oral iron supplementation, or history of adverse or inadequate response to either mode of therapy.
- Hemoglobin response to oral iron therapy is slow, and laboratory tests to evaluate response should not be rechecked until 4 weeks after starting therapy.
- IV therapy is faster, and a 50% response can be seen in 1 week. Repeat hemoglobin levels should be checked at 2-3 weeks.1
- It is important to note that not all patients with iron deficiency develop anemia.1 However, iron deficiency, even in the absence of anemia, is associated with an inability to mount an appropriate erythropoietic response with blood loss. Therefore, all patients with iron deficiency should receive preoperative iron supplementation.1
- Erythropoietin
- Erythropoietin is a glycoprotein hormone secreted by the kidneys that serves as the primary stimulus for RBC production in the bone marrow.1
- Erythropoietin stimulating agents (ESAs) are exogenous forms of erythropoietin produced by recombinant DNA technology. The most common forms are epoetin alfa (Epogen) and the longer-acting darbepoetin alfa (Aranesp).
- ESAs can benefit patients with anemia of chronic disease, chronic renal failure, or patients with IDA that do not respond to iron supplementation alone.1
- ESAs have a black box warning of serious adverse events, including thromboembolism and death in patients with cancer and renal failure. In these patient populations, the risks and benefits of therapy should be carefully weighed.
- For ESA therapy, dosing recommendations vary based on age, creatinine clearance, and reference utilized.
- Therapy should start approximately 3 weeks preoperatively for an optimal response.
Intraoperative Management
- A restrictive transfusion threshold (Hb 7-8 g/dL) has been shown to be equivalent or superior to a more liberal transfusion threshold (Hb 9-10 g/dL).1
- Some of the techniques may not be acceptable to patients in special populations (Jehovah’s witnesses), and these discussions should be addressed in the preanesthetic evaluation.
- Intraoperative strategies to reduce surgical blood loss and address perioperative anemia are listed in figure.2
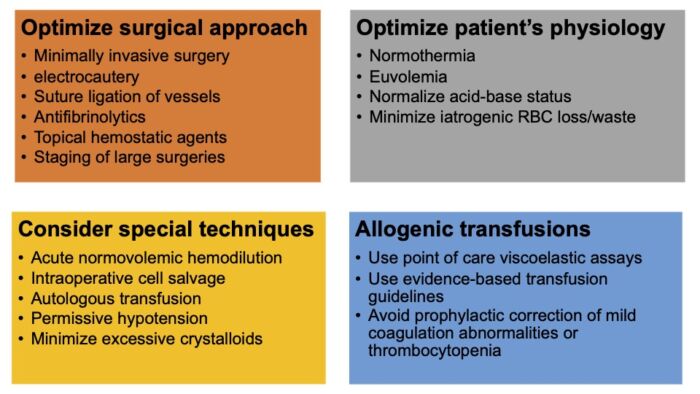
Figure 2. Intraoperative strategies to reduce surgical blood loss and address perioperative anemia. Adapted from Warner MA et al. Anesth Analg. 2020.1
Cell Salvage
- Cell salvage has been used safely in multiple surgical specialties, including, cardiac surgery, major orthopedic surgery, obstetrics, and oncology.
- Absolute contraindications include salvaged blood that is contaminated with bone cement and topical hemostatic agents.
Acute Normovolemic Hemodilution (ANH)
- ANH involves removing blood shortly following anesthetic induction and replacement with a crystalloid or colloid solution. Blood is then infused back into the patient after most of the blood loss has occurred.
- Limitations include preoperative anemia and hemodynamic instability during blood removal.
Postoperative Management
Patient blood management strategies should continue into the postoperative period as approximately 90% of surgical patients without preexisting anemia developed anemia during hospitalization.(Table 1)1
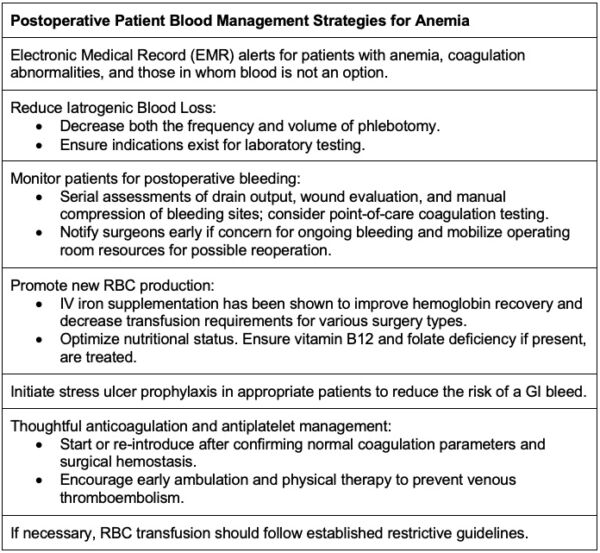
Table 1. Postoperative patient blood management strategies for anemia. Adapted from Warner MA et al. Anesth Analg. 2020.1
References
- Warner MA, Shore-Lesserson L, Shander A, et al. Perioperative Anemia: Prevention, diagnosis, and management throughout the spectrum of perioperative care. Anesth Analg. 2020 May;130(5):1364-80. PubMed
- Hare GMT, Mazer CD. Anemia: Perioperative risk and treatment opportunity. Anesthesiology. 2021;135(3):520-30. PubMed
- Graetz, T, Nuttall, G, Shander, A. Perioperative Blood Management: Strategies to minimize transfusions. In: Post T, ed. UpToDate; 2022. Accessed May 9, 2022.
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.