Copy link
Elevated Intracranial Pressures
Last updated: 08/02/2023
Key Points
- Intracranial pressure (ICP) can fluctuate. An ICP greater than 20-25 mmHg is considered elevated, and an ICP greater than 40 mmHg is severely elevated.
- ICP is mainly affected by the cerebral blood flow, the cerebrospinal fluid volume/flow, and the integrity of the brain parenchyma.
- Acute intracranial hypertension is a neurosurgical emergency, and anesthesiologists can directly employ multiple treatment options for these patients.
Introduction
- ICP normally ranges from 7 to 15 mmHg. It can fluctuate slightly with day-to-day activities. However, a sustained ICP greater than 20-25 mmHg is associated with poor neurologic outcomes, and an ICP above 40 mmHg is life-threatening.1,2
- Most often, a diagnosis of acute intracranial hypertension is made clinically before the direct measurement of ICP.
- Intracranial compliance is defined as the change in intracranial pressure over the change in intracranial volume.1
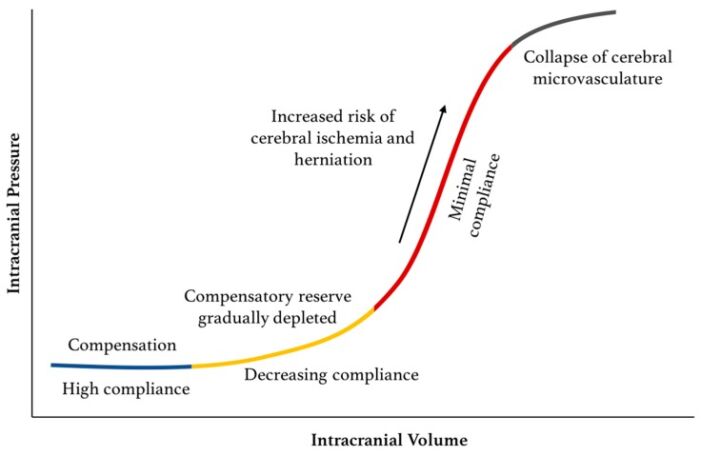
Figure 1. Relationship between ICP and intracranial volume. The pressure-volume curve has four zones. Blue: baseline intracranial volume with good compensatory reserve and high compliance; Yellow: gradual depletion of compensatory reserve as intracranial volume increases; Red: poor compensatory reserve and increased risk of cerebral ischemia and herniation; Grey: critically high ICP causing collapse of cerebral microvasculature and disturbed cerebrovascular reactivity. Source: Hararay M, et al. Intracranial pressure monitoring-review and avenues for development. Sensors (Basel). 2018;18(2): 465.3 CC BY 4.0.
Pathophysiology of Intracranial Hypertension
- The importance of ICP was recognized more than 200 years ago and is referred to as the Monro-Kellie doctrine (or hypothesis), which states that4
- the brain is enclosed in a nonexpandable case of bone;
- the brain parenchyma is nearly incompressible;
- the volume of blood in the cranial cavity is therefore, nearly constant; and
- a continuous outflow of venous blood from the cranial cavity is required to make room for incoming arterial blood.
- The intracranial vault is composed of 3 compartments:
- blood volume
- cerebral spinal fluid (CSF)
- brain parenchyma
- Intracranial hypertension and elevated ICP occur when the volume of one of the compartments increases, and further compensation by a decrease in another compartment is no longer possible.
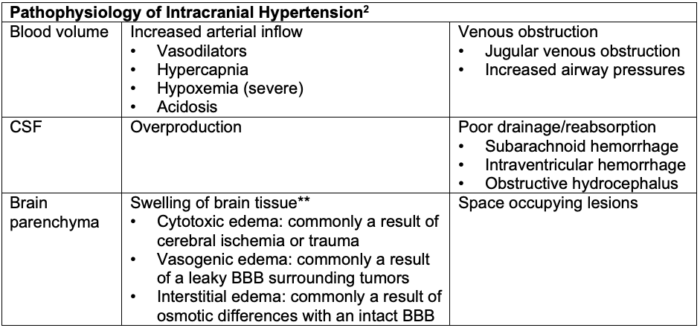
Table 1. Pathophysiology of intracranial hypertension. BBB = blood-brain barrier. **Edema is the most common contributing factor to increased ICP and is commonly multifactorial.
Signs and Symptoms
- Common presenting symptoms of intracranial hypertension include:
- nausea, vomiting, headache, downcast eyes, papilledema, altered mental status, and/or acute focal neurologic deficit
- The concern with acute intracranial hypertension is progression to brain herniation, the symptoms of which would vary depending on the place of herniation.2.3
- The Cushings triad includes hypertension, bradycardia, and irregular respirations. It reflects severe, increased intracranial pressure.1
- The potential sites of brain herniation can be classified into supratentorial and infratentorial (Figure 2).
- Supratentorial: uncal (transtentorial), central, cingulate (subfalcine), transcalvarial, and tectal (posterior)
- Infratentorial: upward cerebellar and tonsillar (downward cerebellar).
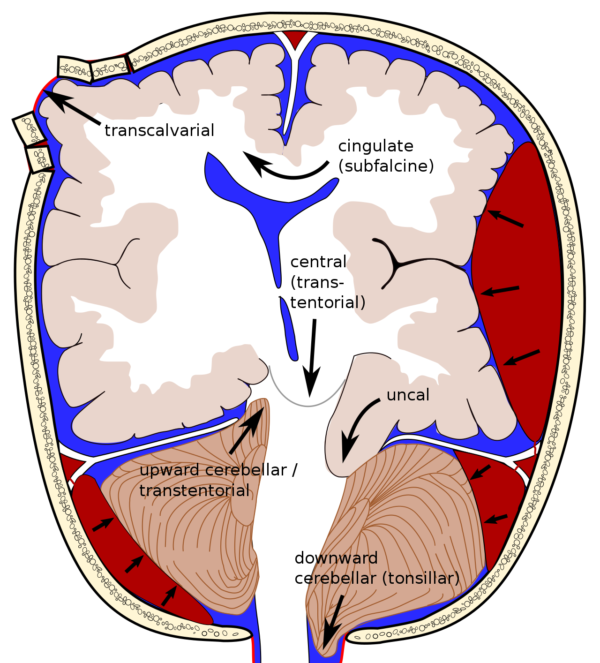
Figure 2. Different sites of possible brain herniation. Cingulate herniation is the most common type. Central (transtentorial) herniation is often fatal in large part due to pulling of basilar artery branches. Source: Wikipedia. Rupert Millard - Brain herniation types.svg by Delldot, CC BY-SA 3.0. Link.
ICP Monitoring
Indications for ICP Monitoring
- Several organizations have proposed guidelines for ICP monitoring. Most generally agree that ICP monitoring is indicated for patients with a structural brain abnormality and a Glasgow Coma Scale (GCS) score < 8.
- Other indications exist regardless of GCS score include:5
- acute brain injuries such as subarachnoid hemorrhage;
- brain abnormalities at risk of rapid progression;
- patients with coagulopathies; and
- patients requiring emergent neurologic surgery.
Modalities for ICP Monitoring
- Invasive modalities
- An extraventricular drain (EVD) inserted into the lateral ventricle is the gold standard. It is both diagnostic and therapeutic as it provides a measurement of ICP and can be also be used to drain CSF as necessary (Figure 3).
- Other invasive modalities include fiberoptic monitoring, combination catheters (“Hummingbird Synergy”) and telemetric monitors. Telemetric monitoring allows the opportunity for long-term ICP monitoring since it involves implantation of the device via a burr hole and reading through an external device.
- Noninvasive modalities
- External structures communicate with the subarachnoid space, and can therefore reflect ICP.5
- Eye: use of ultrasonography to measure optic nerve sheath diameter
- Vasculature: external ultransonography of middle cerebral artery, which is based on the principle that increased ICP affects cerebral blood flow; the vessel wall changes based on ICP and acts as “transducer” of ICP.
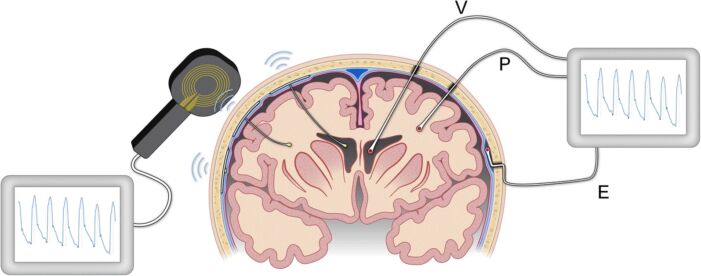
Figure 3. Modalities for ICP monitoring. ICP can be measured via a ventricular (V) catheter placed within the cerebral ventricles, a sensor placed in the brain parenchyma (P), or via a sensor placed in the epidural (E) location. The image on the left illustrates implantable sensors in the ventricles or parenchyma that communicate with an external wireless receiver. Illustration: Øystein Horgmo, University of Oslo. Source: Evensen KH, Eide PK. Measuring intracranial pressure by invasive, less invasive, or non-invasive means: limitations and avenues for improvement. Fluids Barriers CNS. 2020. CC BY SA 4.0. Link.
Management of Elevated Intracranial Pressure by Compartment3
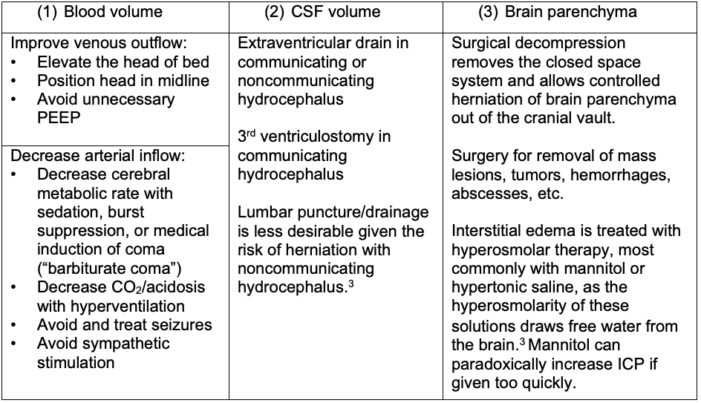
Table 2. Management of elevated ICP by compartment; PEEP = positive end-expiratory pressure
Hyperosmolar Therapy8
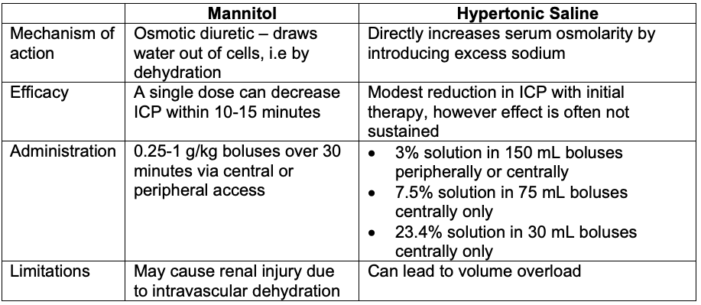
Table 3. Hyperosmolar therapy for elevated intracranial pressures
Cerebral Metabolic Rate of Oxygen Consumption and Burst Suppression
- The brain consumes approximately 3 to 4 mL of oxygen per 100g of brain tissue per minute. This is referred to as the cerebral metabolic rate of oxygen consumption (CMRO2).
- Lowering CMRO2 decreases cerebral blood volume through metabolic autoregulation and subsequent vasoconstriction of cerebral arterioles.
- Burst suppression refers to suppression of cortical electrical activity with the presence of intermittent “bursts” of activity alternating with a flat line as seen on the EEG. This offers neuroprotection by reducing the CMRO2.1
Hyperventilation & its Controversies
- Hyperventilation affects ICP by lowering the arterial partial pressure of CO2, which in turn causes vasoconstriction and decreases cerebral blood flow and volume.
- Increasing CO2 results in arterial vasodilation, and the inverse is true. However, there is a limit to the effectiveness of hyperventilation, such that below a PaCO2 of 25 mmHg, cerebral vasoconstriction has been maximized.
- The brain will adjust to the reduced levels of CO2 within 6-18 hours, and hyperventilation may no longer be beneficial.
- Caution must be taken in patients at risk for cerebral ischemia as the cerebral vasoconstriction will incidentally decrease blood flow to at-risk areas of brain parenchyma.7
- Hyperventilation is associated with worse outcomes in traumatic brain injury.
- Hyperventilation should only be used in cases of impending herniation and as a bridge to more definitive therapy, such as surgical decompression or hyperosmolar therapy.
References
- Bebawy JF, Pasternak JJ. Anesthesia for Neurosurgery. In: Barash PG, et al. Clinical Anesthesia. Eighth Edition. Philadelphia, PA; Wolters Kluwer; 2017.
- Sharma S, Hashmi MF, Kumar A. Intracranial hypertension. In: StatPearls (Internet). Treasure Island, FL. StatPearls Publishing. January 2022. PubMed
- Hararay M, Dolmans RGF, Gormley WB. Intracranial pressure monitoring-review and avenues for development. Sensors (Basel). 2018;18(2): 465. PubMed
- Andrews PJD, Citerio G. Intracranial pressure. Part one: historical overview and basic concepts. Intensive Care Med. 2004; 30:1730-33. PubMed
- DiGiorgio A, Huang MC, Wang VY, et al. Intracranial pressure monitoring. In: Winn HR (ed). Youmans and Winn Neurological Surgery. Philadelphia, PA. Elsevier. 2023. 37, 288-295.
- Anesthesia for Neurosurgery. In: Butterworth IV JF, Mackey DC, Wasnick JD. eds. Morgan & Mikhail’s Clinical Anesthesiology, 7e. McGraw Hill; 2022. Accessed July 18, 2022.
- Monahan C. Intracranial Pressure. In: Freeman BS, Berger JS. Anesthesiology Core Review: Part Two Advanced Exam. McGraw Hill; 2016. Accessed July 19, 2022.
- Ropper A. Hyperosmolar therapy for raised intracranial pressure. N Engl J Med. 2012; 367:746-752. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.